One of the most severe and fatal complications of malaria infection, is cerebral malaria. An improved understanding of cerebral malaria immuno-pathogenesis would greatly improve clinical management and survival rates.
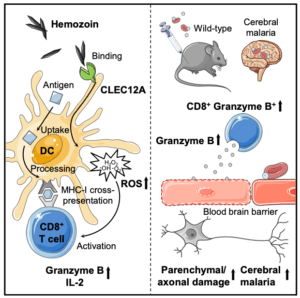
Hemozoin crystals, a by-product of Plasomodium sp. metabolism of haemoglobin in red blood cells, have been shown to accumulate in the brains experimental cerebral malaria (ECM) induced mice. Phagocytic ingestion of hemozoin results in production of pro-inflammatory cytokines and reactive oxygen species (ROS), thus suggesting a potential mechanism of hemozoin induced immuno-pathogenesis. Rualf et al., used a well defined murine ECM models to determine how innate recognition of hemozoin contributes to cerebral malaria pathogenesis.
Toll-like and nod-like receptors have been implicated in ECM pathogenesis, however direct recognition of hemozoin by these receptors has not been demonstrated. Based on findings from other immune-pathogenesis models, C type lectin receptors on myeloid cells have been shown to bind crystals e.g. monosodium urate. Rualf et al., showed that innate cells recognise hemozoin via the myeloid inhibitory C-type lectin-like receptor (CLEC12A). Comparison of ECM immuno-pathogenesis between wild-type and CLEC12A knock out mice, demonstrated reduced ECM pathology and increased survival in CLEC12A KO mice. Reduced symptoms were also associated with reduced ROS, as well as reduced TNF and granzyme B production by both CD4 and CD8 T cells.
Researchers have shown that CD8 T cell release of perforin and granzymes causes brain tissue damage in ECM murine models. Rualf et al., adds to this CD8-mediated mechanism of tissue damage by demonstrating a hemozoin binding of CLEC12A on innate cells induces cross-priming of CD8 T cells via ROS.
Journal Article: Raulf et al., 2019. The C-type Lectin Receptor CLEC12A Recognizes Plasmodial Hemozoin and Contributes to Cerebral Malaria Development. Cell Reports.