After clean water, vaccination has contributed the most to reducing the prevalence of infectious diseases in the 21st century. The most successful vaccines induce durable long-term antibody (Ab) mediated immunity. Induction of such long-lived Abs requires antigen-experienced B cells to interact with follicular helper T (Tfh) cells in the lymphoid tissue facilitating B cell expansion, somatic hypermutation (SHM) and antibody class switching.
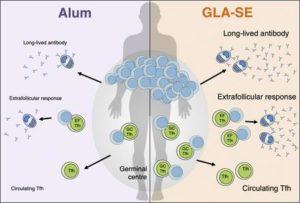
Since Tfh cells play a critical role in the generation of long-live Ab responses, targeting them using adjuvants is a potential way of improving vaccine strategies. Tfh cells are relatively understudied in human vaccine studies because sampling them can be challenging. Studying circulating Tfh (cTfh) cells has been suggested as a suitable alternative because they can be isolated from blood, and phenotypically and functionally resemble Tfh cells. To determine if the type of adjuvant can alter vaccine-induced Ab-immunity in humans, Hill et al., compared malaria-specific Ab immunity in phase 1b vaccine trials. Where Tanzanian male participants were vaccinated with three monthly doses of P27A malaria antigen adjuvanted with either GLA-SE (n=8) or Alhydrogel (Alum, n=8).
To ensure that cTfh cells are a suitable surrogate for studying Tfh cells, Hill et al., showed that cTfh cells in the blood co-express ICOS+CD38+CXCR5+PD-1+ [phenotypic definition of Tfh cells] and positively correlate with the magnitude of vaccine-induced Ab responses. cTfh cells were transcriptionally similar to Tfh cells isolated with the lymphoid organs, and also shared TCRβ clonality. Thus making studying cTfh cells a suitable and more readily available surrogate for understanding vaccine-induced Tfh cells in humans. Hill et al., showed that though both Alum and GLA-SE adjuvanted malaria vaccines induced detectable anti-P27A Ab responses, the GLA-SE adjuvant induced significantly higher proportions of Ab immunity than Alum. Additionally, vaccination with GLA-SE resulted in clonal expansion of TCRβ clones in 5 out of 8 vaccinated individuals. GLA-SE mediated enhancement of Ab immunity was not restricted to the germinal center, but also resulted in increased levels of extrafollicular plasmablast responses (short-lived Ab producing cells).
In summary, Hill et al., demonstrate the type of adjuvant should be a key consideration in vaccine design. Using their study they were able to show that a malaria vaccine “adjuvanted” with GLA-SE induced superior Ab immunity than one “adjuvanted” with Alum. This superior Ab-mediated immunity was attributed to increased levels of cTfh and Tfh cells, and could be harnessed for other vaccine strategies that induced poor Ab immunity.
Journal Article: Hill et al., 2019. The adjuvant GLA-SE promotes human Tfh cell expansion and emergence of public TCRβ clonotypes. Jounral of Experimental Medicine