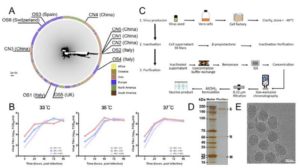
Characterization of PiCoVacc. (A) SARS-CoV-2 maximum likelihood
phylogenetic tree. The SARS-CoV-2 isolates used in this study are depicted with black lines and labeled. The continents where the virus strains were from are colored differently. (B) Growth kinetics of PiCoVacc (CN2) P5 stock in Vero cells. (C) Flowchart of PiCoVacc preparation. (D) Protein composition and purity evaluation of PiCoVacc by NuPAGE 4-12% Bis-Tris Gel. (E) Representative electron micrograph of PiCoVacc. (Source: Gao et al., 2020; Pre-Print)
Disclaimer: This article is a summary of a pre-print by Gao et al., published on BioRxiv. This research article at the time of writing this summary has not been peer-reviewed.
In this pre-peer reviewed article, the authors report on the development of a purified inactivated SARS-CoV-2 vaccine candidate which they show induces virus-specific moderately broadly neutralizing antibodies in mice, rats and non-human primates. A SARS-CoV-2 coronavirus, isolated from a COVID-19 infected patient, was cultured and inactivated with β-propiolactone for 24 hours. After purification and mixing with an Al(OH)3 adjuvant, three intramuscular immunizations were given to macaques at two doses (3 and 6ug/dose). The vaccine was found to provide partial or complete protection (at the higher dose) when subsequently challenged with SARS-CoV-2.
Article: Gao et al., Rapid development of an inactivated vaccine for SARS-CoV-2. BioRxiv Pre-print
Article by Clive Gray










