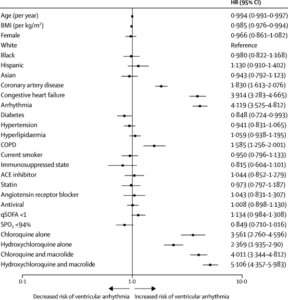
Age and BMI are continuous variables. The 95% CIs have not been adjusted for multiple testing and should not be used to infer definitive effects. ACE=angiotensin-converting enzyme. BMI=body mass index. COPD=chronic obstructive pulmonary disease. HR=hazard ratio. qSOFA=quick sepsis-related organ failure assessment. SPO2=oxygen saturation. (Source Mehra et al., 2020)
Chloroquine was found to be a potent inhibitor of SARS-CoV, the causative agent of SARS, in cell culture in 2005 [1], and was first proposed as a treatment for MERS in 2012 [1]. Chloroquine and it’s analogue hydrochloroquine were proposed as candidate therapeutics against COVID-19, and were one of the drug regiments tested in the World Health Organisation (WHO) Solidarity Trial. However, on the 26th May 2020 the WHO announced the suspension of chloroquine/hydrochloroquine trials for COVID-19. This announcement was made after safety concerns were raised further by a publication by Mehra et al., released in The Lancet on 22nd May 2020.
In their publication, Mehra et al. [2] performed an analysis of the use of chloroquine and hydrochloroquine using registry data from 671 hospitals on six continents. Patients received either chloroquine or hydrochloroquine with or without a macrolide, a class of antibiotics used to treat common bacterial infections. These four groups of patients were compared to a control group that received neither treatment.
All patients were treated within 48 hours of COVID-19 diagnosis . Those who received treatment later than 48h post diagnosis, were on mechanical ventilation, or on remdesivir treatment were excluded. After controlling for confounding factors such as age, sex, disease severity at presentation, and various co-morbidities known to increase COVID-19 morbidity and mortality, the authors found an increase in the risk of death or serious cardiac events (ventricular arrhythmias) in all four treatment groups compared to the control group of patients. When the analysis was performed separately for each continent the findings remained the same.
This study is the largest multinational, observational study on chloroquine/hydroxychloroquine for the treatment of COVID-19 to date, and underscores the need to be cautious when recommending drug regimens that have not undergone randomised, controlled trials. Additionally, these findings were published not long after a chloroquine trial in Brazil was halted early due to several patient deaths in the group randomised to receive higher doses of the drug [3]. Thus further illustrating the risk associated with treating COVID-19 with (hydoxy)chloroquine.
References:
- M. J. Vincent et al., “Chloroquine is a potent inhibitor of SARS coronavirus infection and spread,” Virol. J.,
- M. R. Mehra, S. S. Desai, F. Ruschitzka, and A. N. Patel, “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis,” the Lancet,
- M. G. S. Borba et al., “Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) InfectionA Randomized Clinical Trial” JAMA Network Open
Article by Sherazaan Ismail










