We report recently published results from the phase 1/2 ChAdOx1 nCoV-19 (AZD1222)# vaccine trial, aimed at determining the safety and immunogenicity of a viral vectored vaccine that expresses the SARS-CoV-2 spike protein. Researchers have previously demonstrated that ChAdOx1 vaccines are safe and immunogenic, even in immunocompromised individuals. Additionally, they showed the potential utility of ChAdoX1 nCoV-19 in preclinical studies (Read More).
Trial Design: Healthy adults aged 18–55 years with no history of laboratory confirmed SARS-CoV-2 infection or of COVID-19-like symptoms were randomly assigned (1:1) to receive ChAdOx1 nCoV-19 at a dose of 5 × 1010 viral particles or MenACWY as a single intramuscular injection…. Ten participants assigned to a non-randomised, unblinded ChAdOx1 nCoV-19 prime-boost group received a two-dose schedule, with the booster vaccine administered 28 days after the first dose.
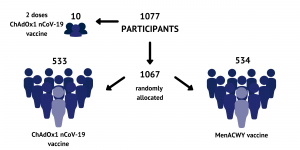
Trial Design (Source: https://www.research.ox.ac.uk/Article/2020-07-20-safety-and-immunogenicity-of-the-chadox1-ncov-19-vaccine-against-sars-cov-2-a-phase-i-2-randomized-control-trial)
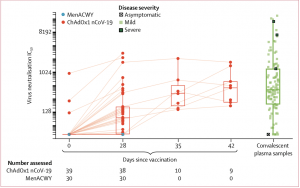
PseudoNA results in trial participants and in convalescent plasma samples from 146 patients with PCR-confirmed COVID-19 and 24 asymptomatic health-care workers. Solid lines connect samples from the same participant. Boxes show median (IQR). Results for days 35 and 42 are samples from participants who received a booster dose at day 28. IC=inhibitory concentration. MenACWY=meningococcal group A, C, W-135, and Y conjugate vaccine. (Source: Folegatti et al., 2020)
Folegatti et al., demonstrated that vaccination with a single dose (prime), induced robust SARS-CoV-2 spike protein-specific antibodies (Abs) which peaked at day 28, and were maintained at similar levels at day 56. As expected vaccination with two doses (prime-boost, 4 weeks apart) resulted in much higher SARS-CoV-2-Ab responses than vaccination with a singe-dose. Researchers observed higher SARS-CoV-2-Ab neutralisation capacity in prime-boost vaccinees compared to prime-only vaccinees. Additionally, prime-boost but prime only vaccination induced Ab-neutralisation capacity in range with those detected in convalescent plasma samples. How long do these responses last ? Based on their experience with other ChAd-Ox vaccine regimens, they speculate that vaccine induced Ab-immunity could be sustained for up to 1 year post-vaccination. What about cellular immunity? Folegatti et al., observed a peak in vaccine-induced T cell immunity (measured by IFNg- ELISPOT) at day 14 post-vaccination, regardless of vaccination strategy, which unfortunately began to decline by day 56.
Authors concluded that “ChAdOx1 nCoV-19 was safe, tolerated, and immunogenic, while reactogenicity was reduced with paracetamol*. A single dose elicited both humoral and cellular responses against SARS-CoV-2, with a booster immunisation augmenting neutralising anti body titres.”
# ChAdOx1 nCoV-19 vaccine (AZD1222) consists of the replication-deficient simian adenovirus vector ChAdOx1, containing the full-length structural surface glycoprotein (spike protein) of SARS-CoV-2, with a tissue plasminogen activator leader sequence.
*In two of the five trial sites (Oxford and Southampton), a protocol amendment (amendment date May 6, 2020) was implemented to allow prophylactic paracetamol to be administered before vaccination and participants were advised to continue with 1 g of paracetamol every 6 h for 24 h to reduce vaccine-associated reactions.
Journal Article: Folegatti et al., 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet
Summary by Cheleka AM Mpande
Also Read: Can a Chimpanzee vector vaccine prevent SARS-CoV-2 pneumonia?










