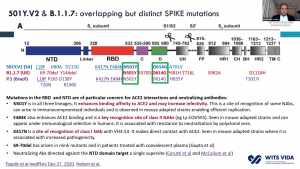
 In the past two-months, preliminary results of the ChAdOx1/nCoV-19 vaccine (AZD1222; phase 2/3) and Ad26.COV2.S candidate COVID-19 (phase 1/2a) suggested that these vaccines were safe and immunogenic, and in the case of ChAdOx1/nCoV-19 had an efficacy of 70.4%. Both vaccines were designed using the genome sequence of the original SARS-CoV-2 strain published in early 2020. Genomic sequencing data have demonstrated the emergence of new SARS-CoV-2 variants that contain multiple mutations in the SARS-CoV-2 spike protein (antigen present in all candidate vaccines) that may affect the efficacy of current vaccines commissioned for emergency use. We recently published a few articles that demonstrate that specific mutations such as K417N, E484K and N501Y present in the B.1.351 variant (first identified in South Africa as early as September) drastically reduce the neutralising capacity of convalescent (from individuals likely infected with the original SARS-CoV-2 variant) and vaccine-induced antibodies.
In the past two-months, preliminary results of the ChAdOx1/nCoV-19 vaccine (AZD1222; phase 2/3) and Ad26.COV2.S candidate COVID-19 (phase 1/2a) suggested that these vaccines were safe and immunogenic, and in the case of ChAdOx1/nCoV-19 had an efficacy of 70.4%. Both vaccines were designed using the genome sequence of the original SARS-CoV-2 strain published in early 2020. Genomic sequencing data have demonstrated the emergence of new SARS-CoV-2 variants that contain multiple mutations in the SARS-CoV-2 spike protein (antigen present in all candidate vaccines) that may affect the efficacy of current vaccines commissioned for emergency use. We recently published a few articles that demonstrate that specific mutations such as K417N, E484K and N501Y present in the B.1.351 variant (first identified in South Africa as early as September) drastically reduce the neutralising capacity of convalescent (from individuals likely infected with the original SARS-CoV-2 variant) and vaccine-induced antibodies.
- Mutations in SARS-Cov-2 B.1.351 variant reduces vaccine induced Ab neutralisation
- Not all SARS-CoV-2 mutations lead to reduced antibody neutralisation capacity
On Sunday 7th February, the South African Department of Health hosted a webinar that highlighted recent findings that have important implications for vaccine roll-out. Watch full webinar below.
In this article, we shall focus on immunological findings presented by Professors Shabir Madhi, Glenda Gray and Salim Abdool Karim.
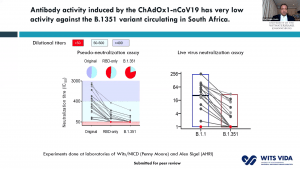 Prof. Shabir Madhi, one of the principal investigators involved in the ChAdOx1/nCoV-19 trial that took place in South Africa, presented data on 2000 participants enrolled in the trial. This vaccine was very immunogenic and induced antibodies in all vaccinated individuals. He demonstrated that before rapid transmission of the B.1.351 variant a single dose of ChAdOx1/nCoV-19 vaccine reduced the risk of acquiring mild/moderate COVID-19 by 75% 14 days post-vaccination. He then discussed factors that contribute to the selection of viruses with mutations (see image). Unfortunately, the ChAdOx1/nCoV-19 vaccine did not achieve the secondary endpoint of the trial: reduction of COVID-19 14 days after 2nd boost. Researchers observed similar proportions of COVID-19 cases in the placebo (n=23) and vaccine (n=19) of which 95% of cases were caused by the B.1.351 variant demonstrating an efficacy of 21.9% (-49.9- 59.8%). Using in vitro studies, they demonstrated that lower efficacy could be due to reduced Ab neutralisation against the B.1.351 variant. T cell profiling suggested that 76/87 known SARS-CoV-2 antigens recognised by T cells are unaffected by mutations in the B.1.351. Prof Madhi then provided data on the Attack rate of seropositive individuals in the Novavax placebo groups
Prof. Shabir Madhi, one of the principal investigators involved in the ChAdOx1/nCoV-19 trial that took place in South Africa, presented data on 2000 participants enrolled in the trial. This vaccine was very immunogenic and induced antibodies in all vaccinated individuals. He demonstrated that before rapid transmission of the B.1.351 variant a single dose of ChAdOx1/nCoV-19 vaccine reduced the risk of acquiring mild/moderate COVID-19 by 75% 14 days post-vaccination. He then discussed factors that contribute to the selection of viruses with mutations (see image). Unfortunately, the ChAdOx1/nCoV-19 vaccine did not achieve the secondary endpoint of the trial: reduction of COVID-19 14 days after 2nd boost. Researchers observed similar proportions of COVID-19 cases in the placebo (n=23) and vaccine (n=19) of which 95% of cases were caused by the B.1.351 variant demonstrating an efficacy of 21.9% (-49.9- 59.8%). Using in vitro studies, they demonstrated that lower efficacy could be due to reduced Ab neutralisation against the B.1.351 variant. T cell profiling suggested that 76/87 known SARS-CoV-2 antigens recognised by T cells are unaffected by mutations in the B.1.351. Prof Madhi then provided data on the Attack rate of seropositive individuals in the Novavax placebo groups 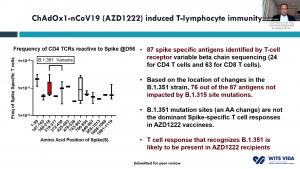 that demonstrated that past infection does not protect against infection with the new variant. Thus, providing evidence as to why the 2nd wave in South Africa exceeded the 1st wave. He further showed that Novavax sub-unit vaccine provided some protection against the B.1.351 variant (efficacy of 49.4%).
that demonstrated that past infection does not protect against infection with the new variant. Thus, providing evidence as to why the 2nd wave in South Africa exceeded the 1st wave. He further showed that Novavax sub-unit vaccine provided some protection against the B.1.351 variant (efficacy of 49.4%).
What will do with the 1 million doses of ChAdOx1/nCoV-19 vaccines already procured by South Africa? Prof Madhi suggested “that the vaccine may still protect against the development of severe COVID-19″ and may warrant providing this vaccine to protect HCW against developing severe diseases.
He ended his talk by highlighting the importance of analysing and monitoring the evolution of the pandemic in real-time, even when some vaccines provide evidence of high efficacy. He highlighted the importance of continuing vaccine trials to determine the effectiveness of vaccines against the new strain, as well as modifying some of the vaccines to improve effectiveness against the B.1.351 strain and other strains that might reduce the effectiveness of the vaccine.
Prof. Glenda Gray then presented results of the Jannsen Ad26 COVID-19 vaccine trial (ENSEMBLE study). Unlike ChAdOx1/nCoV-19, Ad26 COVID-19 vaccine was administered as a single dose. The vaccine had a 66% efficacy (28 days post-vaccination) against developing moderate to severe COVID19 in all regions tested (the USA, Central & South America, South Africa. Unfortunately, the efficacy of this vaccine was 57% in South Africa, with nearly all (93%) cases due to the B.1.351 strain. Based on these results, the South African government is in talks with J & J to provide necessary data required to be approved by the SA medical regulatory body as well as acquire enough doses of the vaccine.
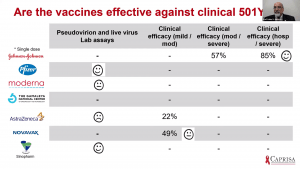
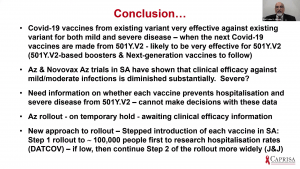
Prof Salim Abdool Karim gave the last presentation, which discussed the implication of vaccine trial results for vaccine roll out in SA. He demonstrated that vaccines with published data [Ad26 (J&J), BNT162b2 (Pfizer/BioNTech); mRNA-1273 (Moderna); ChAdOx1/nCoV-19 (Oxford/AstraZeneca] does show protection against mild to severe COVID-19 due to pre-existing variants they were designed. Based on lab-based assays, clinical efficacy against mild/moderate COVID-19 and severe COVID-19 hospitalisation, it suggests that the vaccine-induced antibodies may have a reduced effect against the new variants that contain N501Y mutations. Overall, all efficacy studies thus far demonstrate a decline in vaccine-induced effectiveness against mild/moderate COVID-19 due to B.1.351, however, Ad26 J& J was associated with an 85% efficacy against severe COVID-19. Based on this and evidence presented by Profs Madhi and Grey, the SA government has decided to temporarily hold roll-out of ChAdOx1/nCoV-19 vaccination till clinical efficacy information is available. The new approach would be a stepwise roll-out of vaccination to assess the hospitalisation rates before vaccination of the next group. This will ensure that vaccines that all rolled out for mass vaccination are effective against severe clinical COVID-19.
Summary by Cheleka AM Mpande