A recent article published in Kidney International highlights novel findings concerning Tertiary lymphoid tissues (TLTs), which are ectopic lymphoid tissues formed due to chronic inflammatory microenvironment which functions as a local center for priming immune response. TLTs are constituted of a hematological portion made up largely of T and B cells, along with stromal components including fibroblasts. TLTs have comparable fundamental functions to lymph nodes and other secondary lymphoid organs.
TLTs can trigger adaptive immune responses and release high levels of proinflammatory cytokines. In addition, certain TLTs generate germinal centers, which are distinct structures in which B cells proliferate quickly and transition classes, culminating in the formation of antibody-secreting plasma cells. TLTs and secondary lymphoid organs have functional similarities, but they also rely on related proteins for development, such as the homeostatic CXC chemokine ligand 13 (CXCL13) and retinoic acid.
The authors have previously reported the TLTs in aged mice kidneys along with diseased and aged human kidneys, although the exact role and formation of TLTs remain unclear. The authors divided TLTs into three separate developmental phases based on the presence of follicular dendritic cells and germinal centers in surgically resected kidneys from elderly adults with or without chronic kidney disease, as well as kidneys resected for pyelonephritis. Sato et al., 2020 also reported that TLTs are distinct from immune infiltrates and reportedly reversed the TLTs in mice kidneys. Interestingly, TLTs in this study were reportedly heterogeneous, complicating the understanding of their role in pathogenesis.
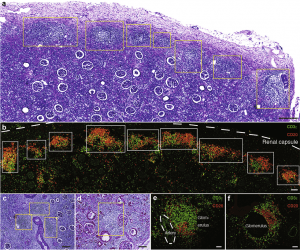
Figure 2: Human pyelonephritic kidneys exhibited multiple lymphocyte aggregates. Histological analysis of human pyelonephritic kidneys. (a,c,d) Periodic acid–Schiff staining showed a chain of aggregates extending over long areas (a) just under the renal capsule and (c) multiple aggregates around blood vessels and (d) in periglomerular areas. (b,e,f) Immunofluorescence of CD3ε (a T-cell marker) and CD20 (a B-cell marker) in areas (b) just under the renal capsule, (e) around blood vessels, and (f) in glomeruli. Yellow and white boxes indicate the localization of aggregates. The white dotted line in part (b) indicates the renal capsule, whereas the dotted line in part (e) indicates the location of an artery. Bars = (a) 300 μm, (b,d) 100 μm, (c) 200 μm, and (e,f) 50 μm (Sato, et al., 2020).
This is a relatively novel study, investigating tertiary lymphoid tissue and how they form and reverse from advanced stages which offers major insights into our understanding of immune response in chronic inflammatory conditions.
Journal article: Sato, Y., et al., 2020. Developmental stages of tertiary lymphoid tissue reflect local injury and inflammation in mouse and human kidneys. Kidney International.
Summary by Gaurang Telang