In a recent review, scientists presented numerous key themes in the biology of pancreatic cancer that can act as identifiers for pancreatic cancer therapy, offering substantial insight into the field of pancreatic cancer research (Figure 1). Genomic changes, metabolism, the tumour microenvironment, immunotherapy, and creative clinical trial design are some of these topics.
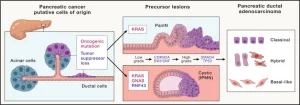
Figure 1: Initiation and progression of pancreatic cancer. Pancreatic ductal adenocarcinoma (PDAC) forms from the exocrine tissue of the pancreas. Acinar and ductal cells have both shown the potential to serve as cells of origin for PDAC upon acquisition of oncogenic mutations and/or loss of tumor-suppressor function. Activating mutations in the oncogene KRAS are found in the two most commonly observed PDAC precursor lesions: pancreatic intraepithelial neoplasia (PanINs) and cystic lesions termed intraductal papillary mucinous neoplasms (IPMNs). In addition to KRAS mutations, activating mutations in the gene encoding for the G-protein alpha subunit Gαs (GNAS) and loss of function of the tumor-suppressor gene RING-type E3 ubiquitin ligase (RNF43) are associated with IPMNs. As these precursor lesions progress from low grade to high grade lesions, loss of the tumor-suppressor CDKN2A or components of the SWI/SNF chromatin-remodeling complexes are observed. Further deletion or inactivating mutations of tumor-suppressor genes SMAD4 or TP53 accompany the advancement of precursor lesions to PDAC. PDAC has also been classified into several RNA-based transcriptomic subtypes. Classic and basal-like have emerged as two consensus groups, with a third “hybrid” capturing those with overlapping features.
Beginning and development – The exocrine tissue of the pancreas is where pancreatic ductal adenocarcinoma (PDAC) develops. Both acinar and ductal cells have demonstrated the ability to act as the PDAC origin cells in the event that they pick up oncogenic mutations or lose their tumor-suppressor role. The two most frequent PDAC precursor lesions, pancreatic intraepithelial neoplasia (PanINs) and cystic lesions known as intraductal papillary mucinous neoplasms (IPMNs), both include activating mutations in the oncogene KRAS.
One of the most difficult and lethal types of cancer is pancreatic ductal adenocarcinoma, which accounts for the great majority of cases of pancreatic cancer. Despite significant advancements in our understanding of the biology of PDAC over the past few decades, the majority of patients’ clinical management has not experienced a significant advance. They think that combined advancements in the areas they have identified as hallmarks will have a revolutionary impact on the way this disease is treated. The authors stress the significance of taking a multifaceted approach to the condition, taking into account as many of the characteristics as is practical for the greatest likelihood of success.
In this review they discuss how improvements in single-cell analysis and high-dimensional spatial profiling techniques have revealed the variety of cell populations dynamically interacting within pancreatic tumours and how to start upsetting these networks to enhance response to treatment.
Journal article: Halbrook., C.J., et al., 2023. Pancreatic cancer: Advances and challenges. Cell.
Summary by Stefan Botha










