Microglia and BAMs are two distinct subsets of macrophage immune cells residing in the brain. These central nervous system-resident macrophages (CRMs) are responsible for the brain’s immune defence against foreign pathogens.
In a recent study, researchers investigating Parkinson’s disease utilized a mouse model to reveal a significant shift in our understanding of neuroinflammatory mechanisms (Figure 1). They found that the neuroinflammatory response in the brain is primarily mediated by border-associated macrophages (BAMs), rather than microglia, which challenges previous assumptions.
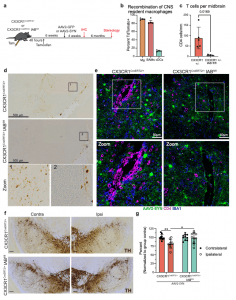
Figure 1: CNS resident macrophage antigen presentation mediates neurodegeneration. a Experimental paradigm for conditional Iab deletion from CX3CR1+ cells. Mice received tamoxifen and 4 weeks later were given AAV2-SYN. Four weeks post AAV2-SYN, flow cytometry and immunohistochemistry were performed. Six months post-AAV, unbiased stereology was performed to quantify neurodegeneration. b Quantification of recombination efficiency of CNS resident macrophages, including microglia (Mg) and border-associated macrophages (BAMs), or meningeal classical dendritic cells (cDCs) 6 weeks after tamoxifen treatment using the CX3CR1CreERT2 mice crossed to TdTomatofl/fl reporter mice. N = 4 mice, unpaired two-tailed T test. *p = 0.0169. Mean ± SEM is displayed. Representative results of two independent experiments are shown. c Quantification of ventral midbrain CD4+ T cells in (d). n = 5 CX3CR1+/− mice and 4 CX3CR1+/− Iab fl/fl mice, **p < 0.01. Mean ± SD is shown. d Representative scans of the ventral midbrain containing the SNpc of DAB staining for CD4+ T cells in CX3CR1CreERT2/+ Iabfl/fl or CX3CR1CreERT2/+ mice. Zoomed images are below showing higher magnification of the boxed area. Numbers indicate if the zoomed image was from CX3CR1CreERT2/+ or CX3CR1CreERT2/+ Iabfl/fl mice. Representative images from two independent experiments are shown. e Immunofluorescent images of CD4+ T cell infiltration in CX3CR1CreERT2/+ Iabfl/fl or CX3CR1CreERT2/+ mice. Brains are labeled with AAV2-SYN (green), CD4 (red) and IBA1 (blue). Images are taken at ×40, scale bar is 50 μm (top) or images are digitally zoomed (bottom). Representative images from two independent experiments are shown. f Representative DAB images of the substantia nigra pars compacta in CX3CR1CreERT2/+ Iabfl/fl or CX3CR1CreERT2/+ mice. Images are labeled with tyrosine hydroxylase (TH), and both the contralateral uninjected (left) and ipsilateral injected (right) sides of the brain are shown. Scale bar is 100 μm. Representative image from one experiment is shown. g Quantification of unbiased stereology of TH+ immunostained neurons in the SNpc of CX3CR1CreERT2/+IABfl/fl or CX3CR1CreERT2/+ control mice who received AAV2-SYN. Quantification is performed at 6 months post AAV transduction and neuron counts are normalized to the average of the group contralateral side. Two-way ANOVA, with Bonferonni multiple comparisons correction, n = 10 mice per group. **p = 0.0053, *p = 0.0105. Mean ± SD is shown. *Source data is provided in the “Source data” file.
The research began by specifically deleting a protein called MHCII in microglia, which typically plays a role in immune responses. Surprisingly, this deletion had no effect on reducing neuroinflammation. This led the researchers to hypothesize that another subset of CRMs, aside from microglia, might be presenting the alpha-synuclein antigen to CD4+ T cells, thereby inducing neuroinflammation and the infiltration of peripheral immune cells from outside the central nervous system. In contrast, when BAMs were selectively depleted, multiple indicators of neuroinflammation were significantly reduced. This discovery suggests that BAMs, rather than microglia, play a crucial role in recruiting peripheral immune cells into the brain parenchyma and re-stimulating antigens, a process responsible for alpha-synuclein-induced neurodegeneration.
Furthermore, the study revealed that the overexpression of alpha-synuclein prompted CRMs to transform into disease-associated microglia and disease-activated BAMs. Parkinson’s disease patients exhibited a significantly higher presence of T cells (both CD4+ and CD8+ T cells) adjacent to BAMs compared to healthy individuals.
This study opens new avenues for investigating and potentially targeting neuroinflammation in the context of Parkinson’s disease.
Journal article: A. M. Schonhoff, et al., 2023. Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nature Communications.
Summary by Stefan Botha










