Pregnancy is a remarkable feat of nature, where a woman’s body undergoes a multitude of changes to accommodate and nurture a growing fetus. One of the adaptations is how the mother’s immune system shifts to protect her developing child, ensuring it is not mistaken for a hostile invader. Recent research has shown that tolerance between the mother and the fetus goes beyond what was previously understood, unveiling a complex and enduring connection that extends well beyond childbirth.
The maternal immune system’s role during pregnancy is akin to that of a vigilant sentinel. It must adapt to provide a nurturing environment for the developing fetus while simultaneously guarding against potential threats. This delicate balance is crucial for a healthy pregnancy and successful childbirth.
In a new study, researchers have demonstrated in mice, the profound and long-lasting nature of maternal immune adaptation during pregnancy (Figure 1). It is already known that a mother’s body becomes more accommodating for a subsequent pregnancy with the same partner. This process involves short-term immune adjustments, but researchers have now uncovered a fascinating long-term component.
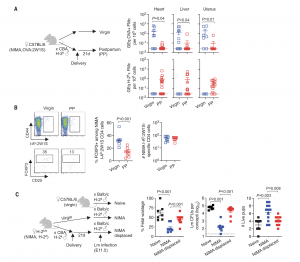
Figure 1: MMcs and NIMA-specific tolerance displaced after pregnancy.(A) Levels of ovalbumin (OVA) DNA specific to OVA:2W1S+MMcs or H-2kDNAspecific to H-2k+FMcs in each tissue among OVA:2W1S NIMA mice 21 dayspostpartum (PP) after allogeneic pregnancy sired by H-2k+CBA males (red) orage-matched 12- to 14-week-old virgin OVA:2W1S-NIMA mice (blue). GEq,genome equivalents. (B) Representative plots and composite data showingFOXP3+among CD4 cells with I-Ab:2W1S surrogate NIMA specificity and numbersof I-Ab:2W1S+CD4 cells in pooled secondary lymphoid organs for the micedescribed in (A). (C) Percent fetal wastage, average recoverable colony-formingunits (CFUs) from concepti in each litter, and numbers of live pups per litter5 days after maternal Lm infection midgestation [embryonic day 11.5 (E11.5)] fornaïve H-2bC57BL/6 mice during primary pregnancy sired by H-2dBALB/cmales (black), compared with genetically identical H-2dNIMA mice undergoingprimary pregnancy sired by H-2dBALB/c males (blue) or H-2dNIMA miceduring secondary pregnancy by H-2dBALB/c males with prior pregnancy byH-2kCBA males (red). Each point indicates the data from an individual mouseand is representative of at least three independent experiments, each with similarresults. Bar, mean ± standard error.
What drives this persistence? Researchers have identified fetal microchimeric cells as the key players. These small populations of cells, originating from the fetus, linger within the mother’s body post-pregnancy. This discovery underscores the extraordinary connection between mothers and their children at a cellular level. What’s known is that a mother can only harbor one set of microchimeric cells at a time, and these cells shift during subsequent pregnancies.
Interestingly, mothers retain the memory of their children, and daughters remember their mothers differently. While the supply of protective fetal microchimeric cells reflects only the most recent pregnancy, a select number of suppressive T cells from each pregnancy remains latent within the mother. These cells can persist for years, waiting to be activated by a new pregnancy. They serve as a backup mechanism, supplementing the role of traditional memory suppressive T cells.
This research opens up exciting possibilities. It suggests that the maternal immune system might remember unfavourable pregnancy outcomes, much like it recalls successful ones. By understanding the immune mechanisms underlying both scenarios, we could develop improved treatments for high-risk pregnancies.
Moreover, this newfound knowledge about the interactions between maternal and fetal cells provides insights that extend beyond reproduction. It could inform advancements in fields such as vaccine development, autoimmunity, and transplantation, shedding light on the intricate ways our immune systems function.
In essence, the story of how mothers remember their babies adds a new dimension to our comprehension of pregnancy and maternal immunity, inviting further exploration into the remarkable mechanisms that sustain the bond between a mother and her child.
Journal article: Tzu-Yu, S., et al, 2023. Reproductive outcomes after pregnancy-induced displacement of preexisting microchimeric cells, Science.
Summary by Stefan Botha










