In a recent study, researchers have unveiled a novel approach to enhance the immune system’s battle against solid tumours. This method, utilizing minuscule nanocapsules, has demonstrated its potential to empower the immune system, leading to more effective cancer immunotherapies (Figure 1). At the heart of this breakthrough lies an innovative technique that augments the number and vigour of immune cells primed to combat cancer cells, ultimately offering new hope for cancer patients.
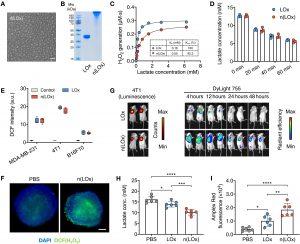
Figure 1: n(LOx) retains the enzyme activity with enhanced stability. (A) Representative transmission electron micrograph (TEM) of n(LOx). Scale bar, 50 nm. (B) Gel electrophoresis of native LOx and n(LOx). (C) Enzyme kinetics of native LOx and n(LOx) in generating H2O2. Kcat, turnover number. (D) Lactate concentration in the culture media of MDA-MB-231 cells after incubating with native LOx or n(LOx) (n = 3). (E) H2O2 generation in the culture media of MDA-MB-231, 4T1, or B16F10 cells after incubating with native LOx or n(LOx) (0.25 μg/ml) for 30 min (n = 5). a.u., arbitrary units. H2DCFDA is used as an indicator of H2O2, which can be oxidized by H2O2 to produce highly fluorescent 2′,7′-dichlorofluorescein (DCF). The fluorescence intensity of oxidized H2DCFDA (DCF) was used to evaluate the generation of H2O2. (F) Fluorescence imaging of intracellular H2O2 in MDA-MB-231 tumor spheroids after incubating with native LOx or n(LOx). H2O2 and nuclei were stained by 6-chloromethyl (CM)–H2DCFDA (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue), respectively. Scale bar, 100 μm. (G) Biodistribution of native LOx and n(LOx) after intratumoral injection. 4T1-Luc cells were used for luminescent imaging. Native LOx and n(LOx) were labeled with DyLight 755 for fluorescence imaging. (H and I) Concentrations of lactate (H) and H2O2 (I) in tumors from mice treated as indicated (n = 6 mice per group). H2O2 was measured with Amplex Red reagent. All data are presented as means ± SD. Statistical significance was calculated by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Solid tumours, notorious for their resilience, emit copious amounts of lactate, creating a hostile environment for the immune system. This hostile setting hampers the immune response’s efficacy against the cancer. Previous strategies aimed at reducing lactate levels relied on drug inhibitors, but these often interfered with the metabolism of healthy cells, causing severe side effects.
In a quest to tackle immune dysfunction surrounding tumours without endangering healthy cells, the researchers conceived a unique tool for delivering drug inhibitors directly to degrade lactate within solid tumours. This innovative solution entailed encapsulating an enzyme, known as lactate oxidase, within tiny nanocapsules designed to diminish lactate levels and unleash hydrogen peroxide within the tumour.
To assess the impact of nanocapsules equipped with the lactate oxidase enzyme, the researchers conducted experiments using mice afflicted with melanoma and triple-negative breast cancer. Their investigations included tumour growth measurements, survival analysis, RNA sequencing, and immune cell population assessments. The results showed an increase in the number and activity of immune cells attacking the cancer.
This study offers fresh hope in the realm of cancer immunotherapy. By utilizing nanocapsules to fine-tune the tumour microenvironment, researchers have laid the foundation for a powerful and precise tool in the fight against solid tumours.
Journal article: Cao, Z., et al., 2023. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Science Translational Medicine.
Summary by Stefan Botha










