A recent study has shown the versatility of Zika virus’ NS2B-NS3 enzyme, potentially identifying a vulnerability that could serve as a therapeutic target (Figure 1). This enzyme, known as NS2B-NS3, serves dual roles—functioning both as a protease and a helicase—crucial for the virus’ replication process.
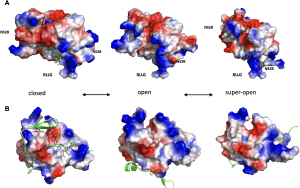
Figure 1: The conformational landscape of ZIKV NS2B-NS3pro. (A) Transitional equilibrium between closed, open, and super-open conformations of ZIKV NS2B-NS3pro. A peptide-based substrate (RKADI, green ball and stick model) in the protease active center is modeled on the related structure of WNV NS2B-NS3pro + aprotinin (PDB ID: 2IJO). The RLLG loop faces the surface of the ER membrane. (B) Same conformations as in B after rotating up by 90 degrees along the horizontal axis. The active site is now facing the viewer. NS2B is shown in green cartoon structure. Positively charged side chains are blue colored, and negatively charged residues are red.
The research unveiled that the Zika virus’ NS2B-NS3 enzyme demonstrates a shape-shifting behavior, adapting its functionality based on its conformation. In its closed conformation, it acts as a conventional protease, cleaving long polypeptides into essential Zika proteins. However, it transitions between open and super-open conformations, enabling it to grasp and subsequently release a single strand of RNA—an indispensable mechanism for viral replication.
As an RNA virus belonging to the flavivirus family, which encompasses deadly pathogens like West Nile, dengue fever, and yellow fever, Zika poses a significant threat, particularly to pregnant women, infecting uterine and placental cells.
Previously, it was known that NS2B-NS3pro, composed of NS2B-NS3pro and NS3hel units, functioned primarily as a protease. However, this study revealed its newfound ability to bind single-stranded RNA and facilitate the separation of double-stranded RNA during viral replication.
Leveraging crystal structures, protein biochemistry, fluorescence polarization, and computational modeling, researchers dissected the lifecycle of NS2B-NS3pro. The enzyme becomes active in the closed conformation, acting as a protease, and transitions through open and super-open conformations to bind and release RNA.
The interplay between NS3pro and NS3hel within the NS2B-NS3pro complex was described, where the grabbing and releasing of single-stranded RNA mimics backward inchworm movements, with the protease component trailing behind.
Apart from offering a potential therapeutic target against Zika, this comprehensive understanding holds promise for tackling other flaviviruses that share similar molecular machinery. This in-depth knowledge of NS2B-NS3pro’s versatile roles could pave the way for targeted interventions against a spectrum of flaviviruses.
Journal article: Shiryaev, S.A, et al, Dual function of Zika virus NS2B-NS3 protease. PLOS Pathogens.
Summary by Stefan Botha










