A major study sheds light on the mysteries of long COVID, a condition affecting millions worldwide. Researchers identified distinct patterns of inflammation in the blood of long COVID patients, offering hope for improved diagnosis and treatment (Figure 1).
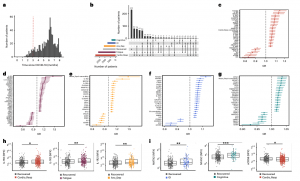
Figure 1: Subtypes of LC are associated with distinct inflammatory profiles. a, Distribution of time from COVID-19 hospitalization at sample collection. All samples were cross-sectional. The vertical red line indicates the 3 month cutoff used to define our final cohort and samples collected before 3 months were excluded. b, An UpSet plot describing pooled LC groups. The horizontal colored bars represent the number of patients in each symptom group: cardiorespiratory (Cardio_Resp), fatigue, cognitive, GI and anxiety/depression (Anx_Dep). Vertical black bars represent the number of patients in each symptom combination group. To prevent patient identification, where less than five patients belong to a combination group, this has been represented as ‘<5’. The recovered group (n = 233) were used as controls. c–g, Forest plots of Olink protein concentrations (NPX) associated with Cardio_Resp (n = 365) (c), fatigue (n = 314) (d), Anx_Dep (n = 202) (e), GI (n = 124) (f) and cognitive (n = 60) (g). Neuro_Psych, neuropsychiatric. The error bars represent the median accuracy of the model. h,i, Distribution of Olink values (NPX) for IL-1R2 (h) and MATN2, neurofascin and sCD58 (i) measured between symptomatic and recovered individuals in recovered (n = 233), Cardio_Resp (n = 365), fatigue (n = 314) and Anx_Dep (n = 202) groups (h) and MATN2 in GI (n = 124), neurofascin in cognitive (n = 60) and sCD58 in Cardio_Resp and recovered groups (i). The box plot center line represents the median, the boundaries represent IQR and the whisker length represents 1.5× IQR. The median values were compared between groups using two-sided Wilcoxon signed-rank test, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
This UK study, the largest of its kind, analyzed blood samples from over 650 hospitalized COVID-19 patients, some of whom developed long COVID. The analysis revealed ongoing inflammation in patients with long COVID, detectable through specific immune system markers in the blood. Interestingly, the exact pattern of inflammation varied depending on the dominant symptom (e.g., fatigue or cognitive impairment).
This discovery suggests that existing medications that modulate the immune system could be repurposed to treat long COVID. With an estimated 65 million people globally suffering from long COVID, the urgent need for effective treatments is clear. This study provides a crucial springboard for further research.
Researchers observed a common theme of immune system activation in long COVID patients. They specifically identified inflammation of myeloid cells and activation of the complement system, both key players in the body’s defense mechanisms. Overactivation of the complement system is linked to various autoimmune and inflammatory conditions.
The study also revealed five distinct long COVID subtypes, each with a unique “immune signature” linked to specific symptoms (e.g., fatigue, cognitive impairment, or gastrointestinal issues). These subtypes suggest that different symptoms may have different underlying causes. This is a significant finding that could pave the way for more targeted treatment approaches.
The researchers propose utilizing these findings to design clinical trials for therapies that target inflammation and specific immune responses. Existing drugs like IL-1 antagonists and JAK inhibitors, used in rheumatoid arthritis and some cancers, could be potential candidates for repurposing in long COVID treatment.
This study brings us closer to understanding the complexities of long COVID and opens doors for the development of personalized treatment strategies.
Journal article: Liew F, et al., 2024. Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease. Nat Immunol. 2024.
Summary by Stefan Botha










