Homeostasis in multicellular organisms can be maintained through various modes of cell death in the form of apoptosis (programmed cell death), necrosis (release of intracellular contents, such as damage-associated molecular patterns – DAMPs, through a raptured plasma membrane induce an immune response) and necroptosis (programmed lytic cell death which integrates both apoptosis and necrosis machinery).
Necroptosis has been reported to be a more immunogenic response compared to necrosis due to its active production of inflammatory cytokines during the cell death phase, often resulting in a more potent activation of T cells. Research into this mechanism of cell death has shown its role in viral infections, and more recently, various inflammatory diseases such as inflammatory bowel diseases (IBD) and neurodegenerative disorders. Optogenetics, which utilizes light-induced protein conformational changes allowing for the modulation of molecular functions and spatiotemporal control of signal transduction in live cells and animals, can activate necroptosis.
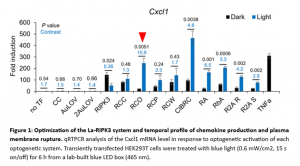
In a new study, researchers developed a light-activatable receptor interacting protein kinase 3 (La-RIPK3) system and performed in vitro and transcriptomics analysis to show that RIPK3 polymerization upregulated genes involved in cellular stress and downregulates those involved in cell survival (Figure 1). They constructed and screened nine photoactivatable RIPK3 association systems, based on their cytokine production and membrane rapture using HEK93T cells, and noted the RCO module had a 10.9-fold upregulation of CXCL1 expression compared to the controls, while the CIBRC module showed the most potent production of chemokines. Evaluation of plasma membrane rupture (which is also a benchmark for La-RIPK3 assessment) showed significant staining in HT-29 cells, indicating membrane rupture.
When an immunofluorescence assay was performed, the authors noted that NIH/3T3 cells expressing mCH-RIPK3 showed less than 10% co-localisation with RIPK1 in both light and dark, however when cells were treated with a necroptosis inducer, this increased the colocalization rate to approximately 60%, while La-RIPK3 activation increased the colocalization rate to 35% compared to cells kept in the dark. The authors also assessed the spatial accuracy for optogenetic induction of necroptosis and noted that majority of the cells underwent necroptosis. Transcriptomics analysis, performed to identify differentially expressed genes in HT-29 cells showed an upregulation of 114 genes in oligomerization-induced La-RIPK3 activation, including genes involved in inflammatory process and oxidative stress.
The findings in this paper highlighted how La-RIPK3 activation can significantly increase DAMP levels and thus optogenetics induction of lytic cell death can enhance the outcome of immunotherapy, especially against solid tumours.
Journal article: Oh ., et al., 2024. Spatiotemporal Control of Inflammatory Lytic Cell Death Through Optogenetic Induction of RIPK3 Oligomerization. Jmb.
Summary by Mbali Mkhonza