- Patient Presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Prevention
- Final Outcome
- MCQs
- References
Patient presentation
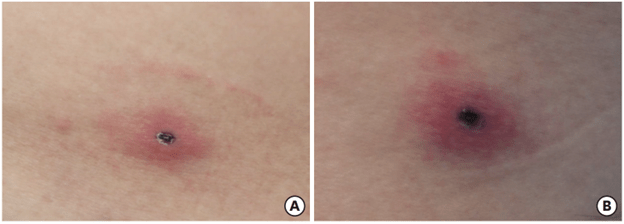
A 36-year-old man developed two eschars (localized skin lesions) after visiting a rural field in Manzini, Swaziland. He has general weakness, myalgia, night sweats and sore throat. He reports having no rash. He has enlarged bilateral inguinal lymph nodes.
Acknowledgement
This case study was created by Immunopaedia Ambassadors Tatenda Murangi, PhD, Post-Doctoral Research Fellow, Fallon Lab, School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin, Ireland and Emmanuel Adamolekun, University of Medical Sciences Teaching Hospital, Akure, Nigeria and Helix Biogen Institue, Ogbomosho, Nigeria.
History
- Fever (38.4C) for 5 days after return from Manzini
- Visited rural field in Manzini, Swaziland for 15 days
Differential Diagnosis
- Malaria
- African tick bite fever (ATBF)
- Influenza
- Scrub typhus
Examination
- No Eschars on abdominal skin and left posterior thigh
- Bilateral inguinal lymph nodes enlarged
- Physical examination of lymph nodes (inguinal and the right popliteal) and a 1cm pustular tender nodule with surrounding erythema and induration in the right popliteal fossa
- Rash absent
Investigations
- On the first at the clinic, slightly decreased white blood cell count (3,600/mm3 )
- Polymorphonuclear cells detected
- Lymphocytes (22%)
- Hemoglobulin (14.5 g/dL)
- Elevated C-Protein ( 22,6 mg/L
- Platelet count ( 198 x 103 mm3)
- A serum sample provided a confirmatory immunoglobulin titer to africae.
Chemistry Examination
- Normal with urea nitrogen of 3 mg/dL
- Creatine (0.75 mg/dL)
- Liver function normal with aspartate aminotransferase level of 29 IU/L
- Alanine aminotransferase level of 20 IU/L
Negative tests
- Blood cultures
- Malaria microscopy
- Rapid antigen test
PCR Tests
Due to presence of eschars, PCR tests were done for:
- Rickettisa
- Anaplasma
- Ethrlichia
- Nested polymerase chain reaction (PCR) using the eschar confirmed ATBF caused by Rickettsia africae.
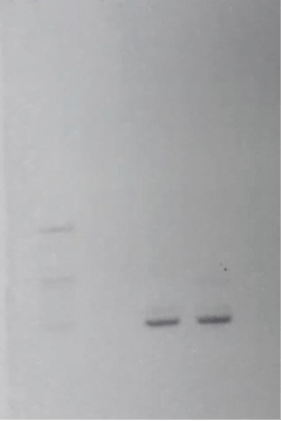
Figure 2. Agarose gel electrophoresis of the amplified DNA fragments. The DNA fragments were obtained by nested polymerase chain reaction (PCR) of the eschar using a primer based on nucleotide sequences of a gene encoding the 17 kDa antigen of Rickettsia africae. A 423-bp DNA fragment was amplified by nested PCR. M, Marker; 1, negative control; 2, positive control; 3, eschar of thigh.
Discussion
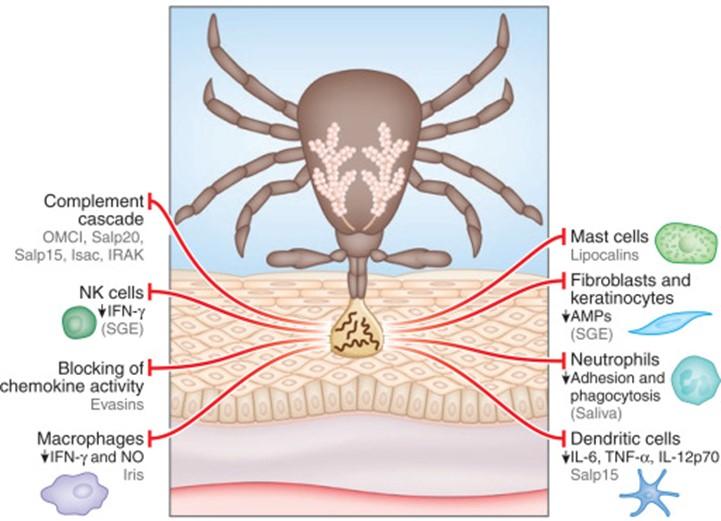
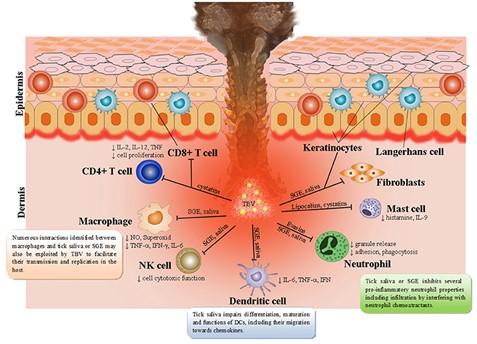
The patient contracted African tick-bite fever (ATBF) caused by Rickettsia africae. This obligate intracellular Gram-negative rod bacterium is transmitted through the bite of ticks from the genus Amblyomma. The immune response involves both innate and adaptive pathways.
Upon transmission, R. africae multiplies at the inoculation site, forming necrotic eschars. The immune response begins with the recognition of the bacterium by pattern recognition receptors (PRRs) on immune cells, such as Toll-like receptors (TLRs). TLR4 and TLR2 likely recognize lipopolysaccharides (LPS) and lipoproteins from the bacterial cell wall. This activation triggers the release of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, recruiting neutrophils and macrophages to the site.
In the adaptive immune response, infected cells present bacterial antigens on MHC class I and II molecules, activating CD8+ cytotoxic T cells and CD4+ helper T cells. The latter aids in differentiating B cells into plasma cells, producing specific antibodies (IgG and IgM) against R. africae. These antibodies facilitate bacterial clearance through phagocytosis by macrophages.
R. africae can evade the immune response by surviving within endothelial cells and delaying seroconversion, especially with early antibiotic treatment, which can suppress aspects of the adaptive immune response.
Clinically, R. africae infection manifests as fever, myalgia, fatigue, headache, regional lymphadenitis, and characteristic eschars. Diagnosis relies on clinical suspicion due to the lack of rapid confirmatory tests and potential cross-reactivity with other rickettsioses. Early initiation of doxycycline is critical to prevent complications such as neuropsychiatric symptoms, encephalopathy, and myocarditis. The immunological pathways in ATBF are crucial for recognizing and clearing R. africae, with prompt clinical intervention necessary to prevent severe complications.
Treatment
- Treated with 100 mg oral doxycycline every 12 hours for 7 days.
- The standard treatment for African tick-bite fever (ATBF) is doxycycline 100 mg twice daily. Alternatives are limited, with chloramphenicol not available in the U.S. Ciprofloxacin and macrolides like azithromycin and erythromycin have been used but with inconclusive efficacy. Josamycin is suggested for pregnant women based on in vitro sensitivity, though clinical data are sparse. In this case, a patient with confirmed ATBF was successfully treated with rifampin, which, despite not being a first-line treatment for rickettsial infections, was effective due to its in vitro susceptibility against Rickettsia africae.
Adjunct therapies
African tick-bite fever (ATBF) includes supportive treatments such as antipyretics to reduce fever, hydration to prevent dehydration, antihistamines for itching, and topical antiseptics for eschars.
Emerging therapies
These aim to enhance diagnostic accuracy and treatment effectiveness, including developing new antibiotics for those intolerant to current options, vaccine development for immunity against R. africae and other rickettsial infections, advanced diagnostic tests like improved PCR and antigen detection methods, and immunomodulatory agents to modulate the immune response, reduce symptom severity, and prevent complications. These strategies aim to reduce ATBF incidence and improve patient.
Prevention
Prevention strategies focus on avoiding tick bites, particularly in endemic areas of Southern Africa, using insect repellents, wearing protective clothing, performing regular tick checks, avoiding tick habitats, and using permethrin-treated clothing.
Final Outcome
The patient recovered without complications.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
References
- Katswara, T., Mukaratirwa, S. Knowledge, attitudes and practices on African tick bite fever of rural livestock communities living in a livestock-wildlife interface area in the Eastern Cape Province of South Africa. BMC Infect Dis 21, 497 (2021). https://doi.org/10.1186/s12879-021-06174-9
- Strand, A., Paddock, C. D., Rinehart, A. R., Condit, M. E., Marus, J. R., Gillani, S., Chung, I. H., & Fowler, V. G. (2017). African Tick Bite Fever Treated Successfully With Rifampin in a Patient With Doxycycline Intolerance. Clinical Infectious Diseases, 65(9), 1582–1584. https://www.jstor.org/stable/26524642
- Lee, W., Seong, H., Kim, J. H., Choi, H., Kim, J. H., Ahn, J. Y., Jeong, S. J., Ku, N. S., Choi, J. Y., Kim, C. M., Kim, D. M., & Yeom, J. (2022). A case of African tick-bite fever in a returning traveler from Southern Africa. Infect Chemother, 54(1), 202-207. https://doi.org/10.3947/ic.2019.0073