- Patient Presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- MCQs
- References
Patient presentation
A 32-year-old male agricultural worker presented with general discomfort and cough with bleeding sputum. He was coughing sporadically in the last 6 months.
This case study was created by Immunopaedia Ambassador Vanessa Amana Bokagne – Eberhard Karl University Tuebingen, Germany and Centre de Recherches Médicales de Lambaréné, Gabon and IUIS Education Online Sub-Committee Member Caroll Beltrán – Immunogastroenterology Lab, Gastroenterology Unit, Medicine Department, Universidad de Chile, Hospital Clinico, Universidad de Chile.
History
- Two months ago, he noticed unintentional weight loss of 6 kg, fatigue, and sporadic high fever, predominantly at night, that he attributed to flu that other co-workers had.
- Personal medical and surgical history: no significant history, vaccinations unknown.
- Smoked cigarettes since the age of 20.
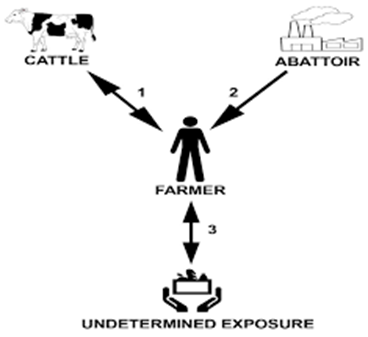
- He worked for 8 years in a slaughterhouse, where cattle bovine tuberculosis (bTB) reactors were also killed.
- Family and social history: He lives with his wife and his children in a cattle farm in Poland, all are healthy. Mother and father’s status is unknown.
- Travel history: No travel outside the country in the last year.
Differential Diagnosis
- Chest X-ray
- Bronchoscopy and biopsy
- Direct Sputum test
- Microbial culture for identification of Mycobacterium sp.
- Influenzae virus
- Histoplasma
- Cryptococcus
Examination
Vitals
- Pulse – 115
- Respiratory Rate – 30
- Temperature – 38°C
General
Slimmed down, fatigued, speaks in a choppy manner due to lack of air
Chest
- Chest shape normal in appearance, tachypnoea present
- Midline trachea
- Bilateral crackles are auscultated bilaterally, wheezing and pectoriloquy at the apex of the right lung.
Cardiovascular
- Tachycardia with a regular rhythm
- Normal S1 and S2, no murmurs
- Bounding pulses felt radially and femorally
- Capillary refill within 2 seconds
Abdomen
- Normal on inspection.
- No rigidity or guarding.
- No organomegaly
- Bowel sounds present
Neurological
Fully awake, alert and co operative
Investigations
| Examination | Value | Normal limits |
|---|---|---|
| WBC | 14 | 4-12 x109/l |
| Hb | 11 | 12.1 – 15.2 g/l |
| Platelets | 170 | 140 – 450 x109/l |
| C-reactive protein | 45 | 0-8 mg/l |
| Respiratory Film-array | Negative | Negative |
| Tuberculin Skin Test | Positive | Negative |
| Direct sputum test for TB | Negative | Negative |
| Incubation in Stonebrink’s medium | Growth of Mycobacterium | No growth |
| MTBC Genotype Test | Mycobacterium bovis | Negative |
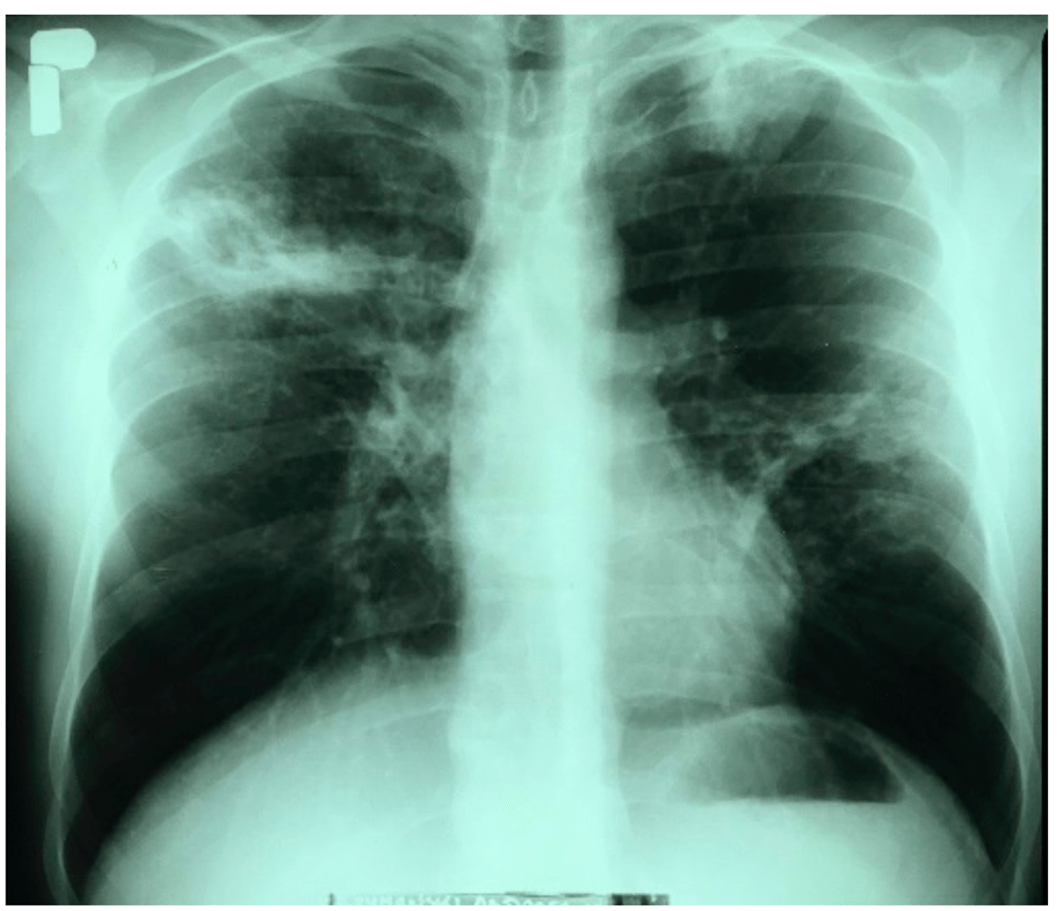
The Chest X-ray of the lungs revealed an infiltrative lesion in the upper field of the right lung, a cavity of approximately 35 × 30 mm, an infiltrative lesion in the left apex, and nodular fibrous lesions in the middle field of the left lung. The left diaphragm and diaphragm-costal angles were without pathological changes. The heart silhouette was within normal limits.
Discussion
This case focuses on a 32-year-old male cattle breeder who was admitted to the Provincial Complex of Healthcare Institutions, Lung Disease Treatment and Rehabilitation Center in Poland because of profuse haemoptysis, which had occurred for the first time in his life. He complained of a persistent cough that had first occurred 6 months prior and for the past 2 months, the cough was accompanied by hoarseness and a high fever (slightly above 39 ◦C), which intensified mainly in the evenings with night sweats. He also suffered from malaise, a lack of appetite, and a weight loss of 6 kg. X-ray image of the lungs was performed and revealed an infiltrative lesion in the upper field of the right lung, with a cavity of approximately 35 × 30 mm, an infiltrative lesion in the left apex, and nodular fibrous lesions in the middle field of the left lung. Suspicions of an infection with pulmonary TB were raised. However, his direct sputum test for TB, acid-fast mycobacteria showed negative results. He was initially put under empiric treatment and supportive care to alleviate the symptoms awaiting further microbiological investigations. After three weeks, incubation on Stonebrink’s medium resulted in the growth of Mycobacterium. Initial identification in the hospital laboratory revealed that the isolate belonged to the Mycobacterium tuberculosis complex which was further confirmed by molecular identification to be Mycobacterium bovis.
M.bovis: Mode of transmission
M. bovis is a bacterium belonging to the Mycobacterium group that causes bovine tuberculous disease in humans and animals. It is a major zoonotic disease, and cattle are the main source of infection for humans. Animal transmission is mainly through aerosol infection and inhalation or, to a lesser extent, exposure to contaminated bodily fluids such as saliva, urine, or feces. Human transmission can also occur through oral ingestion, droplet inhalation or cutaneous penetration. Occupational risks such as working in a farm or abattoir, susceptible age group (infant, pregnant women, old people), consumption related risks (e.g., unpasteurized milk), medical conditions (HIV, diabetes…) are some of the factors that can predisposed infection with M. bovis. In this case, the patient worked for 8 years in a slaughterhouse, where cattle bovine TB (bTB) reactors were killed. It could be possible that he became infected with bTB from exposure to tuberculous animal(s) within the slaughterhouse without following the rules of hygiene and safety while working with infectious material therefore transmitting M. bovis to the cattle on his farm. Another possibility is that he could have been infected by exposure to M. bovis-infected cattle on his farm or by an undetermined exposure (e.g., environmental, ingestion of M. bovis-contaminated dairy products or food, or human-to-human transmission) with transmission to his cattle.
M.bovis: Clinical signs
In humans, symptoms of TB disease caused by M. bovis are like the symptoms of TB caused by M. tuberculosis and include weakness, loss of appetite/weight, fluctuating fever. Coughing and hemoptysis are possible signs and case necrotic, granulomatous lesions within the lung, its associated lymph nodes and those of the head are possible. However, the disease is a spectrum, and its progression varies between individuals and part of the body affected. Most immunocompetent people with no predisposing factor may controlled and usually does not spread the disease as long as the immune system remains strong. In the course of tuberculosis in the described case, the man experienced night sweats, fever, and cough, which are signs of an active TB infection as opposed to latent TB where patient generally shows no symptoms although they harbor the bacteria. Moreover, from the x-ray results, it shows the disease took the form of cavitary pulmonary disease, which is also characteristic of tuberculosis caused by M. tuberculosis. Considering the fact that M. bovis is naturally resistant to the pyrazinamide antibiotic, which is used in the treatment of tuberculosis caused by M. tuberculosis, efficient species identification of the pathogen in this case was very important.
Immunopathology of M.bovis
In cattle, the immune response to M. bovis primarily involves a T cell-mediated immune response. This is often assessed using the tuberculin skin test, which measures the reaction to injected tuberculin purified protein derivative (PPD). Cattle that are infected with M. bovis typically show a strong T cell response, characterized by the activation of T cells and the production of cytokines like interferon-gamma (IFN-γ). In humans, the immune response to M. bovis is quite similar to the response to M. tuberculosis, as both are part of the M. tuberculosis complex. However, the response can vary depending on the virulence of the M. bovis strain and the individual’s immune status.
Immune Response
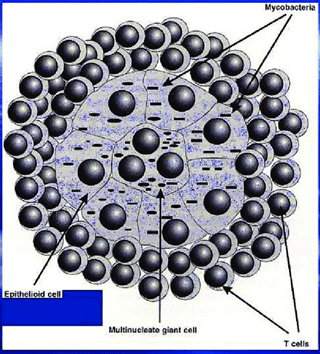
Following exposure, M. bovis enters the human body where it is inhaled and reaches the lungs. The first line of defense involves alveolar macrophages and dendritic cells, which recognize the bacteria through pattern recognition receptors (PRRs). These cells attempt to engulf and destroy the bacteria. However, tubercle bacilli possess the ability to escape killing by preventing phagolysosome fusion and acidification within the phagocytic cells. Mycobacterial lipids such as lipoarabinomannan (LAM) and phosphatidyl inositol mannoside have been shown to intercalate within the endosomal membrane and contribute to the arrest of phagosome maturation. In addition, mycobacterium proteins have been shown to localize within the cytoplasmic vacuoles free of mycobacteria. By this mechanism, mycobacteria survive and multiply within the phagocytes. Following this stage, other phagocytes then enter the area and ingest the increasing number of bacilli. A small cluster of cells referred to as a granuloma develop.
Responses attempting to control the disease result in the accumulation of large number of phagocytes and finally the formation of microscopic lesions denominated tubercles. After 10–14 days, lymphocyte responses develop, and macrophages of the host have an increased capacity to kill the intracellular bacilli. T cells release cytokines that attract, immobilize, and activate additional blood-borne mononuclear cells at the sites where virulent mycobacteria, or their products, exist. The cellular hypersensitivity that develops contributes to cell death and tissue destruction (caseous necrosis). In some instances, liquefaction and cavity formation occur as a result of enzymatic action on proteins and lipids. Cavitation typically occurs during advanced stages leading to hemoptysis as observed on the patient. Rupture of these cavities into the bronchi allows aerosol spread of bacilli. Processing of mycobacterial antigens by macrophages and presentation to T cells play a key role in the release of the appropriate cytokines necessary for full activation of the bactericidal mechanisms of phagocytic cells. Production of IFN-γ by CD4 T cells is an essential component of the immune response to TB (bovine and human). The effector response to mycobacterial antigens is often accessed on diagnostic tests (e.g., IFN-γ production by blood leukocytes via Bovigam®, Quantiferon®, or ELISPOT assays) demonstrating that this response is a good correlate to infection.
Granuloma Formation
To contain the infection, the immune system forms granulomas, which are clusters of immune cells that surround the bacteria. This can prevent the spread of the bacteria but also leads to tissue damage. Granulomas comprise macrophages, T cells, B cells, and fibroblasts. Within granulomas, M. bovis can remain dormant for years, leading to latent TB infection.
Extrapulmonary Disease
In some cases, M. bovis can cause extrapulmonary tuberculosis, affecting organs other than the lungs. This is more common in individuals with weakened immune systems.
Treatment
Total duration up to 6 months
- Rifampicin 600mg
- Isoniazid 300mg
- Pyrazinamide 500mg
- Ethambutol 400mg
- Pyridoxine 50mg daily
Final Outcome
Based on the radiograph of the lungs and the results of the sputum evaluation, the patient was diagnosed with active TB. He was placed under antituberculosis treatment. After 6 weeks of treatment, there was clinical and partial radiological improvement. He was discharged with recommendations for further treatment in a pulmonary clinic and continued his antituberculosis treatment which lasted 6 months.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
References
- Krajewska-W˛edzina, M.; Radulski, Ł.; Waters, W.R.; Didkowska, A.; Zabost, A.; Augustynowicz-Kope´c, E.; Brzezi ´nska, S.; Weiner, M. Mycobacterium bovis Transmission between Cattle and a Farmer in Central Poland. Pathogens 2022, 11, 1170. https://doi.org/10.3390/ pathogens11101170
- Charles O. Thoen, James H. Steele, Michael J. Gilsdorf, Mycobacterium bovis Infection in Animals and Humans. Second edition, 2006