In a new study, researchers have identified a potential breakthrough in preventing heart transplant rejection. Their study focuses on a specific immune response that, while essential for combating infections, also contributes significantly to organ rejection (Figure 1).
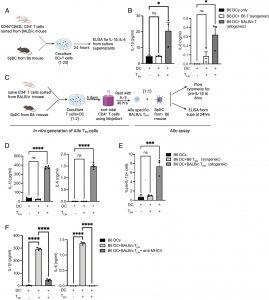
Figure 1: Interaction of CD4+ TEM cells with DCs expressing mismatched MHC molecules leads to IL-1b and IL-6 production. (A) Schematic representation of the DC: T cell coculture system. Briefly, CD62L−CD44+CD4+ T (TEM) cells were magnetically sorted from the spleen and lymph nodes of aged (25 to 35 wk old) BALB/c mice and cocultured at a 20:1 ratio with splenic DCs from B6 mice. Lineage-specific antibodies for T, B, NK cells, erythrocytes, yolk-sac-derived macrophages, and neutrophils (i.e., CD90.2, CD19, NK1.1, TER119, F4/80, Ly6G) were added for depletion to enrich DCs from spleens of B6 mice. Syngeneic TEM cells were sorted from the spleen and lymph nodes of aged B6 mice. (B) IL-1β and IL-6 secreted into the supernatants were measured by ELISA at 24 h. (C) Schematic representation of the experimental setup for in vitro generated BALB/c allo TEM and B6 splenic DCs. (D) ELISA for IL-1β and IL-6 secreted into the supernatants from coculture described in (C) as measured at 24 h. (E) Pro-IL-1β as measured by intracellular staining on the live CD11c+CD11b+ CD90.2− population after 6 h of culture with allo TEM cells. (F) IL-1β and IL-6 as detected and quantified by ELISA at 24 h from cultures of B6 DCs and BALB/c allo TEM cells. Cultures were treated with an MHC-II blocking antibody where indicated. Error bars indicate mean ± SEM. n = 3 biological replicates for (B) and n = 3 to 4 biological replicates for (D–F). Data were generated from three independent experiments for (B) and (D–F). P values were determined by ordinary two-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; ns, not significant).
The immune system’s natural defence against microbial threats involves an inflammatory response. Unfortunately, this same response can also target transplanted organs, leading to their rejection. The team discovered that by blocking a specific signalling pathway between donor dendritic cells and recipient memory T cells, they could significantly reduce this inflammatory response.
In preclinical experiments involving mice, the researchers found that heart transplants in mice genetically modified to inhibit this signalling pathway exhibited prolonged survival compared to untreated controls. This suggests that targeting this pathway could be a promising strategy for preventing heart transplant rejection in humans.
The current standard of care for transplant recipients involves broad-spectrum immunosuppressive drugs, which while effective, increase the risk of infections. The novel approach explored in this study aims to provide a more targeted intervention, blocking only the harmful inflammatory response while preserving the body’s ability to fight infections.
Understanding the precise mechanisms underlying heart transplant rejection has been a significant challenge in the field. By focusing on the role of memory T cells and dendritic cells, the researchers have identified a previously overlooked pathway that may be crucial in developing new therapeutic strategies.
While these findings are encouraging, it is important to note that this research is still in its early stages. Further studies are necessary to determine the safety and efficacy of this approach in humans. If successful, this research could revolutionize the treatment of heart transplant recipients, improving their quality of life and survival rates.
Journal article: Irene Saha, I., et al., 2024. Alloreactive memory CD4 T cells promote transplant rejection by engaging DCs to induce innate inflammation and CD8 T cell priming. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










