Age, which is a major determinant for common chronic disease, involves a progressive loss of protein integrity and function over time, which ultimately results in the progression of diseases and death. Although various diseases, including ischemic heart disease (IHD), stroke, diabetes, and neurodegenerative diseases increase with age, chronological age can be an imperfect measure for the progression of these diseases, and thus the use of omics data may be a better substitute.
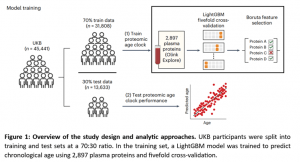
In a new study, blood proteomic information of participants from the UK Biobank (UKB) was selected (and the model was validated against the China Kadoorie Biobank (CKB) and FinnGen) to develop a proteomic age clock to assess 27 aging-related phenotypes (which were related to biological, functional and cognitive status) and 26 age-related diseases (Figure 1).
The authors assessed the association between proteomic aging and aging-related physiological and cognitive function and noted that increased proteomic age gap (ProtAgeGap) was associated with increased levels of kidney function biomarkers, two liver enzymes and decreased levels of albumin. Physical measures of ProtAgeGap showed association with poor self-related health, slow walking pace and frequent insomnia.
Next, the authors determined that proteomic aging is a strong predictor of common diseases. They noted clear differences in ProtAgeGap for ischemic heart disease (IHD), strokes and ischemic stroke as well as for type 2 diabetes when using the CKB. Multivariable Cox proportional hazards were also used to investigate whether mortality in 14 common noncancer diseases persisted after the ProtAgeGap was adjusted for various factors including chronical age, sex etc. The authors noted that ProtAgeGap showed a significant association with mortality in all of the common noncancer diseases studied, except for Parkinson’s (in the UKB cohort).
When examining the 20 proteins selected in the ProtAgeGap20 model, the authors reported that GDF15 was associated with 16 of the 18 diseases studied, indicating that some of the proteins selected in the were associated with multiple major chronic. They also found that the average years of ProtAgeGap increased with multimorbidity.
Thus, the findings highlighted in the study showed consistent associations between ProtAgeGap and a number of cancers and that plasma proteomics may be a powerful tool for the measure of biological age and investigation of common age-related diseases.
Journal article: Argentieri et al., 2024. Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations. Nature Medicine.
Summary by Mbali Mkhonza