A recent publication from the Gattinoni group at the Center for Immunomedicine in Transplantation and Oncology, University Hospital Regensburg, has introduced a new strategy to boost the antitumor response of CD8+ T cells through mitochondrial transfer. This process was first observed as a mechanism for repairing damaged cells. However, in the context of the tumor microenvironment, researchers have discovered that cancer cells can hijack mitochondria from tumor-infiltrating lymphocytes (TILs) to support their growth.
Several mechanisms of mitochondrial transfer (MT) have been identified, including transfer via gap junctions, microvesicles, and the capture of free-floating mitochondria. However, the most prominent method involves the formation of tunneling nanotubes (TNTs). These nanotubes create bridges between neighboring cells, allowing the exchange of cytosolic content, including organelles such as mitochondria.
The Gattinoni group demonstrated that both mouse and human bone marrow stromal cells, when co-cultured with activated CD8+ T cells, can form these nanotubes and transfer mitochondria from the stromal cells to the T cells (Figure 1). This process is driven by genes associated with membrane protrusion, such as Talin2 and Leupaxin.
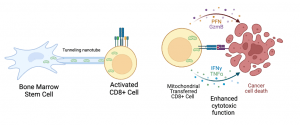
Figure 1: Bone marrow stem cells transfer mitochondria through tunneling nanotubes to activated CD8+ T cells. Mitochondria-transferred CD8+ T cells have enhanced cytotoxic function and are resistant to exhaustion in the tumor microenvironment. (Created in Biorender).
So, what’s the significance of this transfer? When the researchers analyzed T cells that had received mitochondria, they found an increase in metabolic activity, which was linked to enhanced cytotoxic potential. These T cells also exhibited improved proliferation, were less prone to apoptosis, and showed greater resistance to exhaustion in the tumor microenvironment.
To explore the therapeutic potential of this discovery, the researchers generated CAR T cells and co-cultured them with stromal cells to facilitate mitochondrial transfer. The CAR T cells that received mitochondria displayed enhanced cytotoxic capacity.
This breakthrough opens the door to innovative therapeutic strategies, where autologous stromal cells could be harnessed to enhance the metabolic fitness and functionality of T cells, thereby improving their antitumor efficacy. By transferring mitochondria, it may be possible to develop more resilient and effective T cell-based therapies, offering new hope for combating tumors that are resistant to current treatments.
Journal article: Baldwin J.G., et al. 2024. Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Cell.
Summary by Eugenio Contreras Castillo










