Herpes Simplex Virus-1 (HSV-1) is widely known as the culprit behind cold sores, a relatively benign manifestation on the skin. However, its impact extends beyond this superficial presentation, particularly when it invades the central nervous system (CNS). This article delves into the darker side of HSV-1, focusing on its neurological implications, particularly in the brain, and the associated pathological consequences (Figure 1).
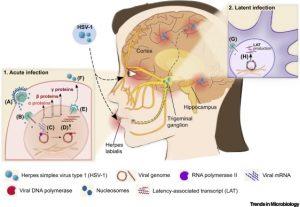
Figure 1. Schematic Representation of Acute and Latent Herpes Simplex Virus-1 (HSV-1) Infection in Humans. Acute infection: (A) HSV-1 virion enters the epithelial cell via envelope fusion with the plasma membrane. Tegument proteins and the capsid containing the linear viral genome are released into the cytoplasm. (B) The capsid reaches the nucleopores by microtubule transport and releases the viral genome into the nucleus, where (C) the genome circularizes and sequential transcription of α, β, and γ genes begins. Arrows indicate nucleocytoplasmic traffic of viral mRNA (black) and related proteins (red). (D) DNA replication begins upon completion of the production of α and β proteins, including viral DNA polymerase. Viral DNA synthesis stimulates the production of γ proteins, which, in turn, participate in capsid assembly and virion formation. (E) The newly synthesized genome is enclosed in the capsid, and the nucleocapsid buds through the nuclear membranes, endoplasmic reticulum, and/or trans-Golgi network vesicles to form an enveloped virion. (F) Newly formed viral particles are released. Latent infection: (G) HSV-1 capsid reaches the neuronal cell body in the sensory ganglia and is transported to the nucleopore by retrograde axonal transport. In the nucleus, viral DNA circularizes and is assembled with the nucleosome, causing the host cell to silence viral genome transcription (H), except for latency-associated transcript (LAT) genes.
The Neurotropic Nature of HSV-1
HSV-1 is a neurotropic virus, meaning it has a predilection for nerve tissues. Following initial infection, typically through mucosal surfaces or skin abrasions, the virus enters sensory neurons and travels retrogradely to the neuronal cell bodies located in ganglia, particularly the trigeminal ganglion. Here, HSV-1 establishes a latent infection, a state in which the virus persists in a dormant form but retains the potential to reactivate.
During latency, HSV-1 expresses a limited set of viral genes, which help the virus evade the host’s immune system and maintain its dormancy. Reactivation can occur due to various triggers such as stress, immunosuppression, or other factors, leading to viral replication and potential spread back to peripheral sites or, more concerningly, into the CNS.
HSV-1 and Encephalitis
One of the most severe outcomes of HSV-1 reactivation in the CNS is herpes simplex encephalitis (HSE). This condition, although rare, is the most common cause of fatal sporadic encephalitis worldwide. HSE primarily affects the temporal lobes of the brain, leading to haemorrhagic necrosis, which is often fatal if left untreated. The clinical presentation includes fever, headache, altered mental status, and focal neurological deficits.
The pathogenesis of HSE involves the virus spreading from the trigeminal ganglion to the brain, where it triggers a robust inflammatory response. This response, although aimed at controlling the infection, often results in significant neuronal damage. The temporal lobes, responsible for functions such as memory and language, are particularly vulnerable, leading to long-term cognitive deficits in survivors.
The Role of Immune Response
The host’s immune response plays a dual role in HSV-1 infections. On one hand, a robust immune response is crucial for controlling viral replication and limiting tissue damage. On the other hand, an overactive or misdirected immune response can contribute to pathogenesis, as seen in HSE.
Research has shown that type I interferons, a group of cytokines crucial for antiviral defense, are pivotal in controlling HSV-1 infection in the CNS. However, the virus has evolved mechanisms to counteract these interferons, such as inhibiting their signaling pathways. This immune evasion allows HSV-1 to persist in the CNS, potentially leading to repeated episodes of reactivation and consequent neurological damage.
HSV-1 and Alzheimer’s Disease: A Potential Link
Recent studies have suggested a possible association between HSV-1 and Alzheimer’s disease (AD), a neurodegenerative disorder characterized by progressive cognitive decline. The hypothesis is based on the detection of HSV-1 DNA in the brains of elderly individuals, particularly those with AD, and the observation that the virus may contribute to the formation of amyloid plaques, a hallmark of AD pathology.
The proposed mechanism involves HSV-1 reactivation in the brain, leading to a localised inflammatory response. This inflammation may promote the aggregation of amyloid-beta, a protein that forms plaques in AD. Moreover, HSV-1 infection has been shown to increase the production of tau, another protein involved in AD, which forms neurofibrillary tangles.
Although this hypothesis is still under investigation, it raises important questions about the potential role of viral infections in neurodegenerative diseases. If proven, it could open new avenues for the prevention and treatment of AD, such as antiviral therapies targeting HSV-1.
Current Therapeutic Approaches and Challenges
The primary treatment for HSV-1 infections, including HSE, is antiviral therapy with drugs such as acyclovir. These medications inhibit viral DNA replication, thereby reducing viral load and limiting damage. In cases of HSE, prompt administration of acyclovir has significantly improved survival rates, although many survivors suffer from long-term neurological deficits.
However, several challenges remain. First, the latency of HSV-1 in neurons poses a significant barrier to complete eradication of the virus. Current antiviral drugs are ineffective against latent infections, meaning that the risk of reactivation persists. Second, the blood-brain barrier (BBB) limits the penetration of many drugs into the CNS, complicating treatment of CNS infections. Lastly, the potential link between HSV-1 and neurodegenerative diseases underscores the need for a better understanding of the virus’s role in long-term neurological health.
Future Directions
Ongoing research is focused on developing more effective treatments for HSV-1, particularly in the context of CNS infections. Novel antiviral agents that can target latent HSV-1 or enhance the host’s immune response to prevent reactivation are of particular interest. Additionally, the potential use of immunotherapy to modulate the immune response in cases of HSE or to prevent the proposed link between HSV-1 and Alzheimer’s disease is an exciting avenue of research.
In conclusion, while HSV-1 is commonly associated with benign cold sores, its potential to cause severe neurological disease should not be underestimated. Herpes simplex encephalitis remains a significant clinical challenge, and the possible link to Alzheimer’s disease highlights the need for ongoing research into the long-term impacts of HSV-1 infection on brain health.
Journal article: Marcocci, M.E., et al. 2024. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Common Virus. Trends in Microbiology.
Summary by Faith Oluwamakinde
References
- Whitley, R. J., & Kimberlin, D. W. (2005). Herpes Simplex Encephalitis: Children and Adolescents. Seminars in Pediatric Infectious Diseases, 16(1), 17-23.
- Wozniak, M. A., Mee, A. P., & Itzhaki, R. F. (2009). Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. Journal of Pathology, 217(1), 131-138.
- Steiner, I., Kennedy, P. G., & Pachner, A. R. (2007). The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurology, 6(11), 1015-1028.










