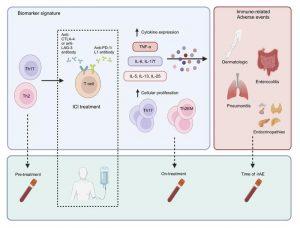
A recent study showed that distinct immune “signatures” in cancer patients undergoing immunotherapy could help predict adverse side effects, enabling earlier interventions to prevent serious complications (Figure 1). This large-scale study is the most extensive research of its kind to date, investigating immune signatures across a broad spectrum of cancers.
As anticipated, about 40% of the participants experienced immune-related adverse events (irAEs). Patients who had these side effects were more likely to have received a combination of immune checkpoint inhibitors or had a pre-existing history of autoimmune diseases. Significantly, the researchers identified a distinct immune signature in those who developed these adverse reactions.
They were able to show that increases in specific types of white blood cells, T-helper 2 (Th2) and T-helper 17 (Th17), and their associated cytokines, appeared before the onset of immune-related adverse events. This shows their therapeutic targeting potential.
While immunotherapy has revolutionized cancer treatment, improving outcomes for many patients, a fraction of those treated with immune checkpoint inhibitors experience severe and sometimes life-threatening side effects. These adverse effects can result in long-term disability, or in extreme cases, death. For patients with advanced cancer who have few remaining treatment options, the benefits of immunotherapy often outweigh the risks. However, the balance becomes more challenging when treating patients with earlier-stage, potentially curable cancers.
Currently, oncologists manage these adverse effects with drugs typically used to treat autoimmune diseases, such as arthritis. However, the exact mechanisms behind the adverse events remain unclear, and optimal treatments are still under investigation. Previous research primarily focused on individuals with skin cancer, often excluding Black patients, who have a lower risk of developing this type of cancer
The researchers found that rising levels of Th2 and Th17 cells served as early indicators of adverse events. In addition, they discovered that higher levels of interleukin 6 (IL-6), an anti-inflammatory cytokine produced by immune cells, strongly predicted which patients would experience immune-related side effects. Elevated IL-6 levels were also associated with poorer outcomes from cancer therapy.
More research is needed to investigate specific immune signatures linked to different types of immune-related adverse events, such as whether the cytokines causing arthritis are the same as those responsible for liver inflammation.
Journal article: Chester J. Kao, C.,J., et al, 2024. Immune-related events in individuals with solid tumors on immunotherapy associate with Th17 and Th2 signatures, Journal of Clinical Investigation.
Summary by Stefan Botha