In recent years, immunotherapy has revolutionised cancer treatment, offering hope for diseases previously considered incurable, such as melanoma, lung, and bladder cancers. By leveraging the body’s immune system to target and destroy cancer cells, immunotherapy has significantly expanded treatment possibilities. However, its efficacy remains limited for many patients due to cancer’s ability to evade immune responses through complex mechanisms, including the creation of an immune-suppressive tumour microenvironment.
In a new study, researchers have identified a previously underappreciated role for monocytes in reactivating T cells within tumours, offering promising new avenues for enhancing immunotherapy (Figure 1).
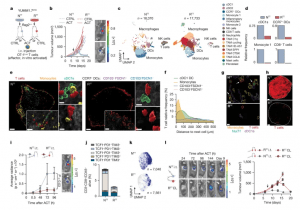
Figure 1: Tumour-infiltrating T cells are restimulated in permissive TMEs. a, Schematic of subcutaneous injection of YUMM1.7OVA NTT cells or RTT cells in Rag2–/– mice and OT-1Luc ACT through intravenous (i.v.) injection. b, Left, ACT responses of NTT and RTT tumours (n = 5 mice per group). Right, representative bioluminescence imaging (BLI) pictures of T cells 96 h after ACT; key indicates radiance (photons per second that leave a square centimeter of tissue and radiate into a solid angle of one steradian). c, Uniform manifold approximation and projection (UMAP) maps of scRNA-seq of CD45+ cells in NTT and RTT tumours 72 h after ACT (n = 4 tumours pooled per group). d, Relative cell frequencies from scRNA-seq. e, Representative multiparameter IF images of YUMM1.7OVA NTT and RTT tumours 48 h after ACT (n = 3 tumours per group). Scale bar, 400 µm (zoom-ins, 50 µm and 10 µm). f, Relative T cell frequency and distance to the next immune cell in NTT tumours (n = 3 tumours per group). g, Representative IF image of Nur77–GFP OT-1 cells in YUMM1.7OVA NTT and RTT tumours 48 h after ACT (n = 3 tumours per group). Scale bar, 10 µm. Arrows indicate Nur77+ OT-1 cells. h, Representative IF image of YUMM1.7OVA NTT and RTT tumours 72 h after ACT. Dashed lines depict the tumour border (n = 2 tumours per group). Scale bar, 500 µm. i, Left, quantification of T cells by BLI over time after intratumoral (i.t.) ACT (mean ± s.e.m., n = 4 mice per group). Two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test. Right, representative BLI image 96 h after ACT (key as in b). j, T cell states by flow cytometry 120 h after i.t. ACT (mean ± s.e.m., n = 5 NTT, n = 4 RTT tumours). k, Effector memory T cell signature29 on scRNA-seq of T cells (n = 14 NTT, n = 18 RTT pooled tumours). l, Left, representative BLI images of T cells in NTT and contralateral (CL) RTT tumours after i.t. ACT (key as in b). Right, growth curves (mean ± s.e.m., n = 6 mice per group). Arrows in b and l indicate day of ACT.
At the heart of cancer immunotherapy lies the “cancer immunity cycle,” a process through which immune cells recognize and eliminate cancer cells. Central to this cycle are T cells, which are activated by antigen-presenting cells (APCs) such as dendritic cells. These APCs capture cancer-derived protein fragments (antigens) and present them to T cells in lymph nodes, priming them for attack. Once activated, T cells travel to the tumour site to destroy cancer cells, restarting the cycle. However, the study highlights a crucial gap in this model: T cells require additional activation upon reaching the tumour microenvironment to sustain their cancer-fighting activity. The team identified inflammatory monocytes as key players in this reactivation process.
Using melanoma mouse models, single-cell RNA sequencing, and advanced genetic and imaging technologies, the researchers investigated why some tumours respond to immunotherapy while others resist. Two melanoma models were studied: one responsive to both immunotherapy and targeted therapy, and another resistant to both.
The responsive tumours were rich in monocytes, a type of immune cell previously overlooked in cancer immunity.
The study also revealed how cancer cells evade immune detection. By increasing prostaglandin E2 (PGE2), a molecule that inhibits both monocytes and dendritic cells, and reducing interferon production, which stimulates immune activity, cancer cells create a microenvironment that suppresses T cell function.
The findings position monocytes as promising targets for enhancing immunotherapy, with potential applications beyond melanoma to cancers like lung, pancreatic, and colorectal cancer. By deepening our understanding of the mechanisms behind anti-tumour immunity, this research represents a significant step toward making immunotherapy more effective and accessible for patients battling cancer.
Journal article: Obenauf, A. et al, 2024. Cancer cells impair monocyte-mediated T cell stimulation to evade immunity. Nature.
Summary by Stefan Botha










