A recent study has identified a novel link between immunosuppression in prostate cancer and a specialized subtype of epithelial cells, known as club-like cells (Figure 1). The research provides new insights into how these cells interact with the immune system, potentially paving the way for improved treatment strategies against treatment-resistant prostate cancer.
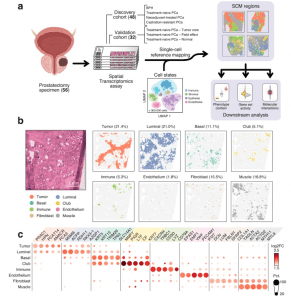
Figure 1: Integration of scRNA-seq and ST data reveals the organizational patterns of the prostate TME. a Sample collection and analysis pipeline overview. Brackets indicate the number of samples in each category. The cell state reference was assembled from previously published single-cell RNA-sequencing data6,7,8,9,12,17,32. Created in BioRender. Nykter, M. (2023) https://BioRender.com/n30i289b) An untreated primary tumor sample with eight SCM regions shown separately. Percentages represent the share of spots across the discovery cohort. For details on how the SCM regions were calculated see Methods. Scale bar is 2 mm. c Expression of cell type gene markers across the discovery cohort. Each region was tested for differentially expressed genes individually in all samples. Dot size represents the percentage of samples in which the gene was overexpressed (Wilcoxon rank-sum test padj < 0.05 & log2 fold change ≥ 1). Dot color represents the average log-fold change. Region-specific marker, and their enrichment test padj (one-sided Fisher’s exact test), and region-specific marker status are indicated in Supplementary Data S5. The number of region-specific markers for each region: Tumor (569), Luminal (1,776), Basal (46), Club (452), Immune (594), Endothelium (139), Fibroblast (294), Muscle (280). SCM regions single-cell mapping-based regions.
Prostate cancer often exploits immunosuppression to evade the immune system, leading to resistance against therapies. This suppression is largely driven by myeloid-derived suppressor cells (MDSCs), which infiltrate the tumour microenvironment, disrupting normal immune responses. Instead of protecting the body, these myeloid cells contribute to chronic inflammation and the accumulation of immune-suppressive signals within tumors. Patients with advanced, treatment-resistant prostate cancer often exhibit high levels of these cells, correlating with a poor prognosis.
The study’s findings highlight the role of club-like epithelial cells in driving this immunosuppressive environment. While these cells originate from the same lineage as prostate cancer cells, they employ distinct signalling mechanisms and are frequently found in regions of tissue with heightened immunosuppressive activity. Importantly, these club-like cells also display resistance to androgen deprivation therapy (ADT), a standard treatment that reduces male hormone levels to inhibit tumour growth.
Sequencing provided a detailed spatial maps of RNA expression within prostate cancer tissue, revealing distinct regions where club-like cells and myeloid cells co-localized. This joint localization was confirmed using mIHC, a method that visualizes proteins with high specificity, validating the findings. These advanced techniques proved essential in understanding how the organization of cells in the tumour microenvironment contributes to immunosuppressive activity.
Treatment resistance remains one of the most significant challenges in managing prostate cancer. The study suggests that targeting the interaction between club-like cells and myeloid cells could offer a novel therapeutic approach. By disrupting these immunosuppressive pathways, it may be possible to enhance the effectiveness of existing therapies and improve outcomes for patients with advanced prostate cancer.
Journal article: Antti Kiviaho et al, 2024. Single cell and spatial transcriptomics highlight the interaction of club-like cells with immunosuppressive myeloid cells in prostate cancer, Nature Communications.
Summary by Stefan Botha










