Malaria remains a critical global health challenge, claiming the lives of over 600,000 people annually, with African children under the age of five disproportionately affected. Severe malaria, caused by Plasmodium falciparum, is particularly deadly, often leading to cerebral malaria and other life-threatening complications. A new study has identified human antibodies capable of targeting key proteins involved in severe malaria, paving the way for novel treatments and vaccines (Figure 1).
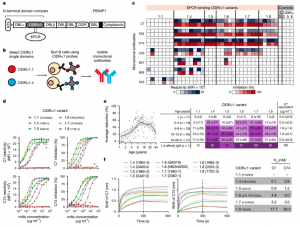
Figure 1: Isolation of mAbs to the PfEMP1 CIDRα1 domain. a, Schematic representation of the CIDRα1-containing multi-domain PfEMP1 proteins, with the N-terminal domain complex comprising the N-terminal segment (NTS), DBLα and the CIDRα1 domain indicated. The EPCR-binding site is also shown. b, Overview of the experimental strategy to isolate mAbs to CIDRα1 domains. c, Heatmap showing mAb reactivity and inhibition of EPCR binding to a panel of CIDRα1 variants (Luminex assay, single measurement). Controls include bovine serum albumin (BSA) and CD36-binding CIDRα2, CIDRα5 and CIDRα6 variants. MFI, median fluorescence intensity. d, Titration of mAb C7 and C74 reactivity (left; single measurement) and inhibition (right; average of four biological replicates) of EPCR binding to CIDRα1 variants representative of each of the six CIDRα1 subclasses. e, Average reduction in plasma IgG reactivity to 19 CIDRα1.4–1.8 protein variants due to competition with C7 Fab plotted against age for each of the 93 individuals (left; with Lowess smoothing curve and 95% confidence interval), and shown as overall averages split by CIDRα1 subclass and age group (right; ±s.d.), with purple shading indicating 25% intervals. Also shown are results for a CIDRα1.4 affinity-purified pool of plasma IgG with broad reactivity (bottom row). The average estimated C7-equivalent plasma concentrations derived from reactivity differentials within each age group are shown (right). ND, not determined. f, Binding curves of C7 and C74 to single CIDRα1 variants (left) and antibody-binding affinity (Kd; right) determined by biolayer interferometry.
Severe malaria arises when P. falciparum infects and modifies red blood cells, causing them to adhere to the walls of small blood vessels, particularly in the brain. This adhesion is driven by a family of highly variable proteins called PfEMP1, located on the surface of infected red blood cells. Specific types of PfEMP1 interact with EPCR, a human protein on blood vessel linings, damaging the vessels and leading to complications such as cerebral malaria.
While immunity to severe malaria develops as children in malaria-endemic regions grow older, the mechanisms underlying this protection have remained unclear. Antibodies targeting PfEMP1 have long been suspected to play a critical role, but the protein’s extreme variability made it a challenging vaccine target. The study overcame this obstacle identifying two human antibodies capable of broadly targeting different PfEMP1 variants. Both antibodies focus on a conserved region of the protein, known as CIDRα1, which interacts with EPCR.
The researchers tested these antibodies’ ability to block EPCR binding in living blood vessels. Further structural and immunological analyses revealed that the antibodies prevent parasite binding by targeting three highly conserved amino acids on CIDRα1. This mechanism likely reflects a natural form of acquired immunity and offers a blueprint for designing PfEMP1-based vaccines or treatments.
This research opens new possibilities for combating severe malaria through vaccines or therapeutic approaches that target PfEMP1. By focusing on conserved regions of the protein, these broadly neutralising antibodies provide a foundation for developing interventions that could benefit a wide range of patients, including those in high-risk regions.
Journal reference: Reyes, R. A., et al. 2024. Broadly inhibitory antibodies to severe malaria virulence proteins. Nature.
Summary by Stefan Botha










