In a new study researchers have developed a computational model that captures the complexities of placental antibody transfer, providing a pathway to more effective and equitable maternal immunisation strategies (Figure 1).
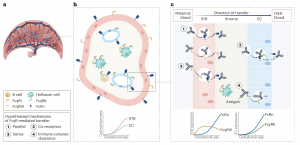
Figure 1: Proposed placental regulators of antibody transfer. a, Macroscopic view of a human hemochorial placenta. b, A single villus cross-section is shown comprising syncytiotrophoblasts (STBs) in pink and fetal capillary endothelial cells (ECs) in blue. The interstitial stroma is shown in tan. Stereotypic Fc receptor expression patterns are shown on each cell type. The cartoon graph depicts expected changes in STB and EC surface area with increasing gestational age. c, Microscopic view of the intervillous space showing the two key cellular layers, STBs and ECs. The direction of antibody transfer is from left to right (maternal to fetal blood). Four hypothesized (but not yet tested) mechanisms of FcγR-mediated IgG transport are depicted. The cartoon graphs show trajectories of Fc receptor expression levels across gestation.
Newborns, with their underdeveloped immune systems, are particularly vulnerable to infections during their first months of life. Maternal vaccines, which provide immunity by transferring protective antibodies through the placenta, play a critical role in safeguarding infant health. However, current maternal vaccination strategies are not optimised for all populations, leaving gaps in protection, particularly for preterm infants and other high-risk groups.
The study outlines a novel framework that focuses on the selective transfer of IgG antibodies, which are crucial for newborn immunity. Unlike traditional empirical vaccine design that relies on static models or animal studies with limited applicability to humans, this dynamic approach integrates the changing structure of the placenta and maternal immune responses over time. The model reveals the detailed kinetics of antibody transfer, pinpointing bottlenecks that may hinder the efficient delivery of antibodies to the fetus. By addressing these barriers, researchers aim to design maternal vaccines that deliver the precise antibodies needed to protect newborns from specific infections. This approach holds promise for improving outcomes for all infants, including those born prematurely, who often miss out on the full maternal antibody protection provided during a full-term pregnancy.
The study emphasises the importance of tailored immunisation strategies for vulnerable populations. Premature infants, for example, are at higher risk of infections due to incomplete antibody transfer during gestation. By understanding the factors that regulate placental antibody transfer, such as maternal antibody levels, placental structure, and fetal development, the model could guide the development of vaccines specifically designed to address these challenges. Beyond newborns, this computational framework has potential applications for other high-risk populations, including immunocompromised individuals and the elderly.
One of the most promising aspects of this computational model is its potential to pave the way for personalised vaccine strategies. The framework can account for individual patient characteristics, such as genetic background, exposure to stressors, or underlying health conditions, allowing for the development of vaccines tailored to specific needs.
For maternal vaccines, the insights provided by this model could lead to the development of vaccines that optimise the natural process of antibody transfer through the placenta. By strengthening this transfer, vaccines could provide newborns with enhanced immunity during their critical early months, reducing neonatal mortality and improving global infant health outcomes.
Although the computational model is currently a research tool requiring further validation, its potential clinical applications are significant. Once refined, the framework could guide patient-specific vaccine strategies, enabling healthcare providers to design immunization plans that maximize efficacy while addressing individual vulnerabilities.
Journal article: Wessel, R.E., et al. 2024. Regulators of placental antibody transfer through a modeling lens. Nature Immunology.
Summary by Stefan Botha










