Chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis (ME/CFS), has long puzzled researchers with its debilitating symptoms and unclear origins. In a new study researchers provide critical insights into the condition, revealing that pathogen-killing CD8+ T cells, a vital part of the immune system, show signs of exhaustion in patients with ME/CFS. This discovery highlights immune dysregulation as a key feature of the disease and opens avenues for potential therapies targeting immune exhaustion.
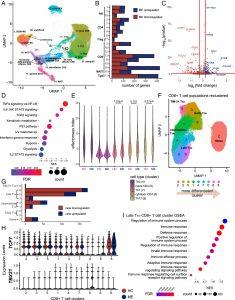
Figure 1: scRNA-seq analysis of T cells in ME. (A) scRNA-seq of T lymphoid cells from 28 cases and 30 controls, depicted by uniform manifold approximation and projection (UMAP). Cluster numbers assigned independent of cluster size. (B) Number of differentially expressed genes (y-axis) per cluster (x-axis), as log2 fold change > 0.25, adjusted P-value < 0.05 (FDR). (C) Volcano plot of differentially expressed genes in γδT cells (cluster 11). Colored dots indicate increased expression in case (blue) or control (red; P ≤ 0.05, log2 fold change > 0.25). (D) GSEA dot plot of γδT cells against hallmark pathways from Molecular Signature Database. Size indicates number of leading-edge genes; color indicates adjusted P-value (FDR). (E) Violin plot showing the distribution of effector index per cell in CD4+ T cell clusters and biological condition (control, ME). (F) scRNA-seq analysis of CD8+ T cells from 28 cases and 30 matched controls, depicted by UMAP. (G) Number of differentially expressed genes per CD8+ T cell cluster, defined as log2 fold change > 0.4 and adjusted P-value < 0.05 (FDR). (H) Violin plots showing differential expression between case and control in each cluster for two exhaustion-associated TFs—TCF7 and TBX21. (I) GSEA of cluster 6 against gene ontology: biological processes terms. Positive values (x-axis) indicate enrichment in cases and vice versa. Dot size and color indicate number of genes in the leading edge, and adjusted P-values (FDR), respectively.
The study identified CD8+ T cells as a focal point of immune dysregulation in ME/CFS patients. These T cells are typically responsible for detecting and eliminating infected or malignant cells. However, in ME/CFS, these cells exhibit markers of chronic stimulation, leading to an “exhausted” state. This phenomenon, well-documented in cancer, occurs when immune cells are continuously activated over a prolonged period, reducing their effectiveness.
The presence of these exhaustion markers suggests that T cells in ME/CFS patients may be responding to a persistent stimulus, such as a viral protein or another chronic immune challenge. This chronic activation not only diminishes the immune system’s ability to fight pathogens but may also contribute to the persistent fatigue and systemic dysfunction characteristic of ME/CFS.
T cell exhaustion is not unique to ME/CFS. Similar immune dysregulation has been observed in patients with long COVID, pointing to potential shared mechanisms between the two conditions. Additionally, the phenomenon is well-studied in cancer, where therapies have been developed to reverse T cell exhaustion and reinvigorate the immune response. These parallels raise the intriguing possibility that anti-exhaustion therapies used in oncology could be repurposed for ME/CFS.
Despite these findings, the underlying cause of T cell exhaustion in ME/CFS remains unclear. Researchers aim to determine whether a persistent viral infection or another immune stimulus drives this chronic activation. Understanding the root cause is critical for designing targeted therapies that address the condition’s underlying mechanisms.
This discovery represents a significant step forward in understanding ME/CFS and its complex relationship with immune dysregulation. By drawing parallels with cancer and long COVID, researchers have identified promising targets for intervention. If therapies to reverse T cell exhaustion prove effective, they could offer much-needed relief to patients suffering from ME/CFS.
Journal article: Iu, D.S., et al., 2024. Transcriptional reprogramming primes CD8+ T cells toward exhaustion in Myalgic encephalomyelitis/chronic fatigue syndrome. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










