Researchers have made significant progress in understanding how to optimize chimeric antigen receptor (CAR) T-regulatory cells (Tregs), a promising avenue for treating autoimmune diseases, improving organ transplant outcomes, and potentially combatting cancer (Figure 1). The study explores how modifying CAR signalling in Tregs can enhance their effectiveness while minimising unintended inflammatory consequences.
Tregs, a subtype of immune cells, play a crucial role in maintaining immune balance by preventing overactive responses that could damage the body. Unlike CAR-T cells, which are engineered to target and destroy cancer cells, CAR Tregs are designed to suppress immune responses and curb inflammation.
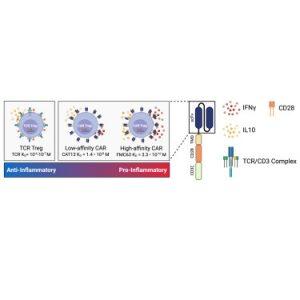
Despite their potential, CAR Tregs have struggled to deliver consistent results. The engineered receptors used in CAR Tregs often fail to mimic the natural signalling processes of these cells, inadvertently promoting pro-inflammatory responses—counterproductive to their intended function. The study,identifies a key factor influencing these outcomes: the affinity of the CAR, or how tightly it binds to its target.
The researchers found that reducing the binding affinity of the CAR significantly improved Treg performance. While lower-affinity CARs still effectively suppressed immune responses, they reduced the secretion of pro-inflammatory cytokines, a harmful side effect of high-affinity binding. This fine-tuning of CAR affinity offers a potential solution to the challenges that have hindered CAR Tregs in clinical applications.
CAR Tregs has diverse potential applications. In autoimmune diseases like type 1 diabetes, where the specific immune trigger remains unknown, CAR Tregs could still be directed toward general inflammatory targets. The study also explores alternative targeting strategies for CAR Tregs, such as focusing on extracellular matrix components or directly modulating aggressor T cells and antigen-presenting cells. These approaches could further enhance the precision and effectiveness of CAR Treg therapies.
By addressing the challenges of CAR Tregs and refining their design, this research brings the field closer to unlocking the full potential of these powerful immune cells, offering hope for more effective and personalized treatments.
Journal article: Cochrane., R.W., et al, 2024. High-affinity chimeric antigen receptor signaling induces an inflammatory program in human regulatory T cells, Molecular Therapy – Methods & Clinical Development.
Summary by Stefan Botha