Psoriasis is driven by an overactive immune system, with dendritic cells (DCs) playing a central role in triggering inflammation. A new study has identified a key protein, NF-kB c-Rel, that plays a significant role in exacerbating psoriasis, an inflammatory skin disease affecting millions worldwide (Figure 1). The research demonstrates that when activated by immune system signals, c-Rel contributes to the severity of psoriasis symptoms. However, its absence alleviates skin inflammation, pointing to a potential new therapeutic target for the condition.
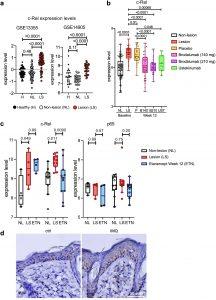
Figure 1: c-Rel expression is increased in the skin of human patients with psoriasis and IMQ-induced psoriasis-like mouse model. (a) c-Rel levels in different public GEO datasets of healthy, nonlesional, and psoriatic lesional skin biopsies (GSE13355, GSE14905). (b, c) c-Rel and (c) p65 levels in psoriatic lesions in patients treated with different biologics (GSE117468, GSE11903). Box and whisker plots tails are defined as 1.5 times interquartile range (IQR) from the first and third quartiles of each dataset. (a–c) Each point is representative of mean expression with 95% CI (One-way ANOVA, Tukey’s multiple comparisons test). (d) Representative immunohistochemistry staining of c-Rel in control or IMQ-treated dorsal skin of C57BL/6J mice at day 5, n = 3 (magnification: 60×, scale bar = 50 μm).
Researchers investigated how c-Rel, a transcription factor in the NF-kB family, influences immune responses in psoriasis, particularly through its interaction with Toll-like receptor 7 (TLR7). TLR7 is a key regulator of innate immunity and inflammation, known to be activated by viral infections and other immune challenges. The study found that higher levels of c-Rel were present in psoriasis patients, suggesting its direct involvement in disease progression. In a mouse model mimicking human psoriasis, mice lacking c-Rel were significantly protected from developing psoriatic symptoms, displaying reduced skin inflammation and fewer disease-associated markers.
The findings highlight an important connection between c-Rel, TLR7 signaling, and psoriasis inflammation. Since TLR7 can be activated by viral infections, including HIV, human papillomavirus (HPV), and hepatitis C virus (HCV)—all of which have been linked to psoriasis—the research suggests that viral infections may worsen psoriatic disease through this immune pathway.
By blocking c-Rel activity, researchers believe it may be possible to reduce inflammation and alleviate psoriasis symptoms.
The study paves the way for further investigation into TLR7-c-Rel-dependent mechanisms that regulate dendritic cell function and their role in worsening psoriatic disease. Understanding how viral infections interact with these pathways could explain why some patients experience more severe psoriasis symptoms after infections.
Journal article: Angela Rose Liu., A.R., et al, 2024. NF-κB c-Rel is a critical regulator of TLR7-induced inflammation in psoriasis, eBioMedicine.
Summary by Stefan Botha










