Rheumatoid arthritis (RA) is an autoimmune disease driven by complex interactions between immune cells in the synovium, the tissue lining the joints. A study published in Nature Communications (2024) sheds new light on how clonal expansion of T and B cells in the synovium contributes to RA progression. By using single-cell RNA sequencing (scRNA-seq) and immune receptor sequencing, the researchers mapped out the clonal relationships between lymphocytes in the synovium and blood of RA patients.
Read Further: Possible new treatment for rheumatoid arthritis
Study Design and Results
The study involved 12 seropositive RA patients, from whom synovial tissue and blood samples were collected. The researchers performed scRNA-seq on over 80,000 cells, focusing on T and B lymphocytes. They identified several clonally expanded populations of T cells, particularly CD4+ T cells, that were enriched in the synovium. Notably, the study highlighted the presence of T peripheral helper (Tph) cells and T follicular helper (Tfh) cells, both of which provide critical support to B cells.
Tph cells, which express chemokine receptors such as CCR2 and CCR5, were found to migrate to inflamed joints, where they help sustain inflammation by promoting B cell activation. The study also found that synovial plasma cells shared clonal B cell receptors (BCRs) with other B cell subsets, indicating that these cells are part of a continuous clonal expansion driven by antigenic stimulation.
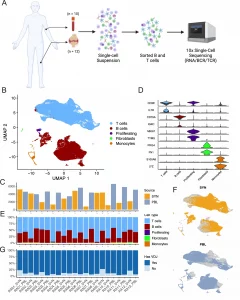
Figure 1: Sorting and single-cell analysis of matched blood and synovial tissue T cells and B cells.
Key Insights
One of the most significant findings was the identification of highly oligoclonal CD8+ T cells in the synovium. These cells, expressing granzyme K (GZMK), are thought to contribute to synovial inflammation by producing inflammatory cytokines. The researchers also found that expanded CD8+ T cell clones were distributed across various transcriptomic clusters, suggesting that they play multiple roles in the inflammatory process.
Additionally, receptor-ligand analysis predicted interactions between Tph cells and B cells through cytokines like IFN-γ and TNFRSF members. These interactions likely drive the chronic inflammation characteristic of RA, particularly in the synovium.
Implications for RA Therapy
This study provides new insights into the cellular interactions that sustain synovial inflammation in RA. The identification of clonally expanded Tph and CD8+ T cells suggests that these populations could be targeted in future therapies. By disrupting the interactions between T and B cells, it may be possible to reduce synovial inflammation and slow the progression of RA.
Moreover, the study’s findings highlight the importance of targeting specific immune cell populations in RA, particularly those involved in antigen presentation and cytokine signaling. Therapies that inhibit Tph cells or the cytokines they produce could offer a new approach to managing RA.
This research deepens our understanding of how clonal expansion of lymphocytes in the synovium drives RA progression. The study’s identification of Tph and CD8+ T cells as key players in RA pathogenesis opens new avenues for targeted therapies, with the potential to improve outcomes for patients suffering from this chronic disease.
Journal Article: Dunlap, Garrett, et al. “Clonal Associations between Lymphocyte Subsets and Functional States in Rheumatoid Arthritis Synovium.” Nature Communications.
Summary by Faith Oluwamakinde










