Type 1 diabetes, which affects approximately nine million people worldwide, is caused by an autoimmune attack on insulin-producing islet cells in the pancreas. Islet transplantation has shown promise in restoring insulin function, but current FDA-approved methods are limited by invasive procedures, immune rejection, and short-term effectiveness.
A new approach to islet transplantation has demonstrated long-term diabetes reversal in mice by incorporating engineered blood vessel-forming cells (Figure 1). The findings suggest that reprogrammed vascular endothelial cells (R-VECs) can significantly improve islet survival and function, potentially paving the way for a safer and more effective treatment for type 1 diabetes.
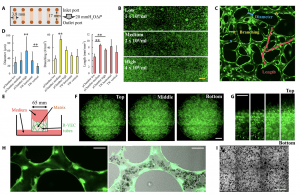
Figure 1: R-VECs form perfusable blood vessels in microchambers and TransWells. (A) A diagram illustrating the size and setup of the microchamber for vessel formation. (B) Perfusable vascular networks are formed by human R-VECs within the microchamber. Vessel networks show different morphologies with low, medium, and high densities of R-VECs. Scale bar, 1 mm. (C) Examples of manually labeled vessel diameters (blue), branching (yellow), and length (red) are shown in a representative enlarged image of the microchamber vasculature. The image measures 1 mm × 1 mm. (D) Quantification of average vessel diameter, branching number per mm2, and average vessel length per mm2. TW, TransWell. “**” above a line indicates P < 0.01 between two columns; “**” on the top of a column indicates P < 0.01 compared to all of the other columns. All data are presented as mean ± SD. (E) A diagram illustrates the size and setup of the TransWell for vessel formation. (F) Microscopic images taken from the bottom of the TransWell show the horizontal-spanning vasculature formed by R-VECs. Scale bar, 1 mm. (G) Microscopic images taken from the side of the TransWell show the vertical-spanning vasculature formed by R-VECs. Scale bar, 1 mm. (H) Heparinized human peripheral whole blood passes through the R-VEC–formed vessels. Scale bar, 40 μm. (I) Red blood cells (shown as black dots) are evenly distributed throughout the vessel network. Scale bar, 1 mm. The green fluorescence shown represents GFP positive ECs in panels (B), (C), (F), (G) and (H).
The current approach involves injecting islets into the liver, where they disperse uncontrollably, lack proper support, and often fail within a few years. Researchers aim to develop a more controlled and long-lasting solution, ideally implanting islets under the skin in a vascularized, immune-protected environment.
To overcome these challenges, they developed R-VECs, a special type of blood vessel-forming cell derived from human umbilical vein cells. These engineered endothelial cells are designed to:
– Provide immediate oxygen and nutrients to transplanted islets
– Form stable vascular networks to sustain islet function
– Adapt to surrounding tissue environments, mimicking natural islet endothelial cells
Key Findings:
- Subcutaneous Transplantation Success
- Mice transplanted with islets plus R-VECs under the skin quickly connected to the host’s blood circulation, ensuring long-term survival.
- The R-VECs adapted to support islet cells, forming a dense vascular network and expressing genes similar to natural islet endothelial cells.
- Long-Term Diabetes Reversal
- Diabetic mice regained normal blood glucose levels and body weight for over 20 weeks—suggesting a permanent islet engraftment.
- In contrast, mice that received islets without R-VECs showed poorer glucose control and shorter transplant survival.
- Potential for Scalable Drug Testing
- The study also demonstrated that islet-R-VEC transplants can be successfully grown in microfluidic devices, allowing for high-throughput drug testing for diabetes therapies.
If successful in future trials, this approach could transform diabetes treatment, allowing long-term insulin independence without the complications of current transplantation methods. The researchers are optimistic that clinical translation could be within reach in the next several years.
Journal article: Ge Li, R., et al. Vascularization of human islets by adaptable endothelium for durable and functional subcutaneous engraftment. Science Advances.
Summary by Stefan Botha










