The introduction of the cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy, Elexacaftor-Tezacaftor-Ivacaftor (ELX/TEZ/IVA), marked a significant breakthrough in the treatment of cystic fibrosis (CF). However, concerns regarding the potential for depression-related adverse events emerged after its approval. A study published in the American Journal of Respiratory and Critical Care Medicine systematically reviewed data from clinical trials, post-marketing reports, and registry-based studies to assess the impact of ELX/TEZ/IVA on depression-related events in people with CF (pwCF).
Additional Reading: Disease-specific Transcriptional Programs Govern Airway Goblet Cell Hyperplasia in Asthma and Cystic Fibrosis
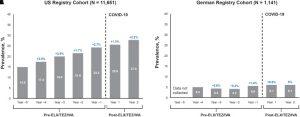
- Figure: Prevalence of depression reported in the elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) postauthorization safety study (PASS) longitudinal cohort before and after initiation of ELX/TEZ/IVA use. (A and B) Results using data from people with cystic fibrosis (pwCF) in the U.S. Cystic Fibrosis Foundation Patient Registry (A) and the German Cystic Fibrosis Registry (Mukoviszidose eV) (B). Time periods include 5 years before ELX/TEZ/IVA initiation and 2 years (Year 1 and Year 2) after starting ELX/TEZ/IVA treatment. Change in prevalence of depression from the previous year is indicated in blue. COVID-19 = coronavirus disease.
The findings from clinical trials showed that the rates of depression-related events, including suicidal ideation and attempts, were comparable between patients treated with ELX/TEZ/IVA and those receiving placebo. Specifically, the exposure-adjusted rate of depression-related adverse events was 3.32 per 100 person-years (PY) in the ELX/TEZ/IVA group, similar to 3.24 per 100 PY in the placebo group. Moreover, post-marketing data from over 61,000 patients revealed low rates of depression-related events, further suggesting that these events are not directly caused by ELX/TEZ/IVA treatment.
Importantly, the study also acknowledged the high background prevalence of depression in pwCF, which is estimated to be two to three times higher than in the general population. This highlights the need for continuous mental health monitoring in this population, irrespective of CFTR modulator therapy. The review concluded that depression symptoms in pwCF treated with ELX/TEZ/IVA appear consistent with the overall epidemiology of depression in the CF population and do not suggest a causal relationship between the treatment and depression-related events.
These findings are crucial for clinicians managing CF patients, as they provide reassurance that ELX/TEZ/IVA, a highly effective therapy for improving lung function and reducing exacerbations, does not significantly increase the risk of depression beyond what is expected in the CF population. The study supports the continued use of this therapy with careful mental health screening as part of routine care.
Summary by Faith Oluwamakinde