In a major advancement for cell-based cancer therapies, researchers at have demonstrated that a novel natural killer (NK) cell therapy, pre-complexed with a bispecific antibody called AFM13 (acimtamig), delivers striking response rates in patients with refractory CD30-positive lymphomas (Figure 1).
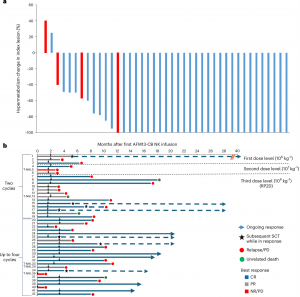
Figure 1: Evaluation of patient responses to AFM13-NK cell therapy. a, Waterfall plot showing treatment responses of individual patients, with CRs defined based on functional imaging as PET negative status (−100% hypermetabolism change in the index lesion). Hypermetabolism was assessed using the maximum standardized uptake value. Each bar represents an individual patient, with blue bars representing HL and red bars indicating NHL. b, Duration of responses to AFM13-NK therapy, both with and without a consolidation SCT. Downward arrows, time of the last cycle of AFM13-NK cells for each patient. Patients with T-NHL are noted on the y axis. The number of treatment cycles that each patient received is indicated next to the y axis. NR, no response.
The therapy uses cord blood-derived NK cells that are activated with cytokines, expanded ex vivo, and then pre-complexed with AFM13 – CD30 on lymphoma cells and CD16A on NK cells
This double-targeting mechanism redirects NK cells to seek out and destroy CD30-positive cancer cells more efficiently.
The study enrolled 37 patients with Hodgkin lymphoma and five with T-cell lymphoma, all of whom had received a median of seven prior lines of treatment. Patients were given two to four cycles of chemotherapy, followed by AFM13-NK infusions across three dose levels and weekly infusions per cycle.
Importantly, the AFM13-NK therapy demonstrated an excellent safety profile:
- No cytokine release syndrome (CRS)
- No neurotoxicity (ICANS)
- No graft-versus-host disease (GvHD)
- Only one Grade 2 infusion reaction reported
This approach highlights a shift toward universal, donor-derived NK cell therapies, which may overcome key limitations of T cell-based treatments, such as manufacturing time and toxicity. With further validation, this strategy could revolutionize treatment for patients with refractory lymphomas and beyond, opening a new chapter in the field of cellular immunotherapy.
Journal article: Nieto, Y., et al., 2025. Banerjee, P., Kaur, I. et al. Allogeneic NK cells with a bispecific innate cell engager in refractory relapsed lymphoma: a phase 1 trial. Nat Med.
Summary by Stefan Botha










