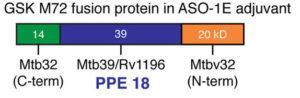
Schematic showing the position of PPE 18 (Mtb39a) in the M72 GSK vaccine. Source: Brennan. Infection and Immunity 2017.
The first ever United Nations High Level meeting on Tuberculosis (TB) (UNHLMTB) was held today (26th September). This meeting represents the acknowledgement and commitment of world leaders to strengthen action and investments towards the fight against TB.
Development of an efficacious vaccine that contributes to control of Mycobacterium tuberculosis (M.tb) infection and progression to TB disease has been identified as one of the tools required to achieve a world without TB. Currently, the only licenced tuberculosis vaccine BCG, confers limited efficacy against TB diseases in adolescents and adults. Highlighting the need for new improved TB vaccines that are protective post-adolescence.
On the eve of the UNHLMTB, results from the Phase 2b M72/AS01E vaccine trial were published in the NEJM. This study represents the first TB vaccine that is able to provide 54% protection against pulmonary tuberculosis diseases in individuals already infected with M.tb.
M72/AS01E vaccine contains the recombinant M72 fusion protein of two M.tb antigens M.tb32A and M.tb39A combined with AS01E adjuvant (the same adjuvant used in the malaria candidate vaccine RST,S AS01). Numerous phase 2(a) trial have been conducted (see Van Der Meeren et al., Supplementary Table S1 below). These studies have shown that M72/AS01E is safe and highly immunogenic. Briefly, M72/AS01E has been shown to induce polyfunctional Th1 CD4 T cells in both M.tb infected and un-infected HIV- individuals, as well as HIV+ individuals. Additionally, the vaccine has been shown to induce robust humoral responses.
Overall, the study published in NEJM represents a positive step forward in the fight against TB. It suggests that a vaccine that only has two M.tb antigens has the potential confer protection against pulmonary TB in M.tb infected individuals (in a TB endemic region).
*M.tb infection was based on positive QuantiFERON-TB Gold In Tube assay result, which measure immune reactivity to M.tb antigens CFP-10, ESAT-6 and TB7.7 antigens absent in BCG.
Journal Article: Van Der Meeren et al., 2018. Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis. The NEJM.
Also See: Bloom 2018. New Promise for Vaccines against Tuberculosis. NEJM
Article by Cheleka AM Mpande
Journal Articles Listed in the Table Below.
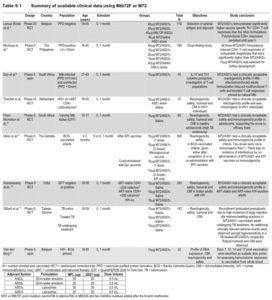
Table S1 Summary of available clinical data using Mtb72F or M72. Source Van Der Meeren et al., 2018 NEJM. Supplementary Material.










