To give perspective on why the development of new vaccines against Tuberculosis (TB) is important, TB is the only infectious disease that is one of the top 10 causes of mortality worldwide. BCG, the only licensed vaccine against TB, is highly effective against disseminated forms of TB in infants. However, variable efficacy has been observed in adolescents and adults, who represent the age group with the highest burden of TB. An estimated 1.7 billion individuals are infected with Mycobacterium tuberculosis (M.tb), of which an estimated 10% (170 million) individuals are expected to develop TB in their lifetime (in 2016). It is estimated that TB cost the global economy approximately $9.2 billion (USD) to care for TB patients, and in some countries such as Germany, treatment of one multi-drug -or extremely drug resistant- TB patient can be between €82, 000 – 109, 000. A vaccine that is capable of preventing M.tb infection or progression to TB disease can alleviate the current burden of the TB epidemic. Additionally, a TB vaccine could also have the potential to improve TB therapy, especially in individuals with various forms of drug resistant because protective immunological responses induced against M.tb should not be affected by drug-resistant M.tb strains.
The Global Forum on Tuberculosis (TB) Vaccines aims to showcase the full spectrum of issues relevant to TB vaccine research and development ranging from basic science research to clinical trials, access and advocacy, to name a few.
This year the 5th Global Forum on TB vaccines took place in New Delhi, India, one of the top three countries burdened with high rates of TB. The conference provided a wonderful platform on which the Indian government and research community to showcase its commitment to achieving the third United Nations Sustainable Development Goal (SDG3) of a 90% reduction of TB deaths and 80% reduction of TB incidence by 2030, compared with 2015. In fact, they have an internal deadline to achieve SDG3 by 2025. They aim to achieve this by improving delivery of health care specifically to TB patients, through various initiatives such as compensation for time lost due to clinic visits during TB treatment and improved communication between private and governmental clinics/hospitals, among others. In addition developing new vaccines against TB, that are aimed at preventing M.tb infection, TB disease as well as improve M.tb therapy outcomes and prevent TB re-occurrence. Repurposed leprosy vaccine, Mycobacterium indicus pranii vaccine (Mw) is an example of a vaccine to that is currently being tested for use during therapy and prevention of TB re-occurrence.
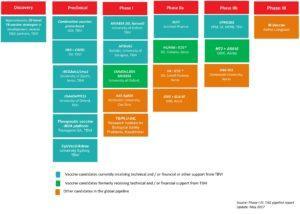
There are currently fourteen TB vaccine candidates that are in Phase I-III clinical trials, of which only one, M.Vaccae is currently in Phase III. We previously, highlighted research on MTBVAC presented at the conference, and we shall highlight more advances and interesting results of vaccine trials and studies that were showcased at the conference, including a talk on Mw.
Visit the 5th Global Forum on TB Vaccines Website to know more
Article: Global Report on Tuberculosis Vaccines 2018.
Article by Cheleka AM Mpande