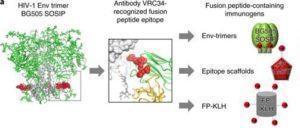
Xu et al., 2018. Figure 1a: tructure-based design, antigenic characteristics, and EM structure of FP immunogens. The glycosylated structure of the HIV-1 Env trimer is shown at far left, with exposed N terminus of FP highlighted in red. Subsequent images show site recognized by VRC34.01 antibody, schematics and antigenicity of FP immunogens, and negative stain EM of FP-KLH (see Supplementary Fig. 1 for details of FP antigenicity). For the EM study, n = 3 independent experiments were performed with similar results.
One of the goals in developing an effective, global HIV vaccine is to elicit potent antibodies, capable of neutralizing the majority of circulating HIV strains. These antibodies are known as broadly neutralizing antibodies (bNAbs), however, to date, HIV vaccines have not successfully elicited these desired responses. In this article, Xu and colleagues from the Vaccine Research Center, USA, developed an immunogen that was capable of eliciting bNAbs in mice, guinea pigs and rhesus macaques. This immunogen will soon be adapted for human trials.
One of the recently discovered epitopes for bNAbs on the HIV Envelope is the fusion peptide. The fusion peptide, 15-20 amino acid residues, is a hydrophobic epitope that is well conserved among HIV strains because it plays a role in viral entry into host cells. The fusion protein was not previously thought to be a highly immunogenic site, as it is buried in certain HIV Envelope conformations and therefore researchers focused on other bNAb epitopes for epitope-based immunogen design. However, the isolation of a bNAb which targets this site, known as VRC34.01, shifted the focus to this epitope.
The researchers, led by Peter Kwong, developed an immunogen which consisted of the HIV Envelope protein and the exposed N terminus of the fusion peptide coupled with a carrier. They immunised mice, guinea pigs and rhesus macaques and found that in all animal models the immunogen elicited bNAb responses. Monoclonal antibodies were purified from the animals after vaccination and in vitro assays revealed that these antibodies could neutralize up to 31% of viruses in a panel of 208 diverse HIV strains.
Altogether, this study reveals that bnAbs can be elicited through immunisation with a fusion peptide-based immunogen. However, the researchers did find low consistency in breadth and potency of elicited antibodies between vaccinated animals. Thus, before testing in humans, breadth and potency of vaccine induced bNAb requires improvement in order for it to be efficacious in humans.
Journal Article: Xu, K. et al., 2018. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nature Medicine
Article by Thandeka Moyo










