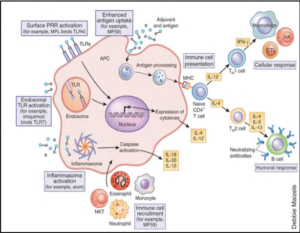
The innate immune system, the first line of immune defence, is activated from minutes to hours after immunization with vaccine vectors and adjuvants. This activation can modulate the quality and quantity of the long-term immune memory responses leading to protection. The laboratory of Dr Erica Andersen-Nissen and others researchers assess the innate immune responses to different HIV vaccine vectors and adjuvants in order to define signatures of adaptive immune correlates of risk or protection.
The innate signatures for 4 HIV recombinant vaccine vectors (MRKAd5/HIV, Canarypox vector (ALVAC), Modified-vaccinia Ankara (MVA) and Vesicular Stomatitis Virus (VSV)) and their respective adjuvants have been evaluated or are under evaluation in order to predict the adaptive responses.
The vaccination with ALVAC and a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E) regimen, reffered to as RV144 vaccine has shown 31% of efficacy (S.Rerks-Ngarm et al, NEJM 2009). This modest but evident partial efficacy offer impetus for immune-correlates analysis for this vaccine. In 2012, Haynes et al, have shown that RV144 vaccine induces HIV-1 gp70-V1V2 binding IgG and Env-specific IgA antibodies and generated the hypothesis that high levels of Env-specific IgA antibodies may attenuate the effects of protective IgG antibodies. By using computational framework for unbiased combinational polyfunctionality analysis of antigen-specific T-cell subsets, Lin et alhave shown that polyfunctional Ag-speficific CD4+ T cells might have a role in RV144 vaccine efficacy (Lin et al, Nat Biotech 2015).
Currently, Andersen-Nissen et al.,are investigating the innate responses that predict correlates of risk in RV144 vaccine in South Africa. Their results currently suggest that vaccines induces robust innate responses as Type 1 IFN signaling as early a day post vaccination. They are currently correlating these early immune responses to vaccine-induced adaptive immunity such as HIV-specific-IgG and IgA binding responses.
Moreover, other ongoing analysis focus on early response signatures predicting correlates of risk or protection in other HIV vaccine vectors (MVA, VSV, Ad5) vaccination but the preliminary data are contrasting.
Profiling early innate immune responses to vaccination allows rapid identification of biomarkers and signatures to determine vaccine immunogenicity or efficacy and give advantages for rational design of new vaccine vectors and adjuvants.
Article by Abdouramane Camara, IBMC, Strasbourg-France