Antiretroviral therapy (ART) can successfully suppress HIV viral load resulting in a functional but not permanent cure in HIV-positive individuals. One of the strategies to achieve permanent cure is the kick-(shock)-and-kill strategy. This strategy is proposed to leverage immune activation -using latency reversing agents (LRAs)- of resting cells with integrated HIV provirus, followed by elimination of these infected cells by effector molecules such as CD8 T (and natural killer) cells. The use of LRAs aims to induce expansion and proliferation of resting cells with integrated HIV genome, allowing protein expression and antigen presentation of HIV antigens that can be targeted by immune cells.
Clinical trials of the kick-and-kill strategy have not yet been successful. The lack of efficacy observed in such trials is potentially attributed to the complexity of natural HIV reservoirs, which is shaped by years of ART, features not reflected in vitro latency models of kick-and-kill strategy. This highlights the need to extend latency models to include ex-vivo CD4 T cells from HIV+ individuals, to develop successful kick-and-kill strategies.
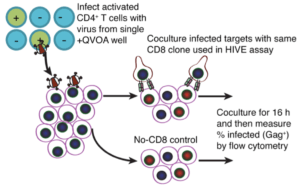
Huang et al., utilised ex-vivo CD4 T cells to determine whether co-culture of effector CD8+ T cells with LRAs could drive reduction of latent HIV reservoir from ex-vivo CD4 T cells from ART treated individuals. Researchers observed a reduction in the level of HIV DNA in response to treatment of ex-vivo CD4 T cells with LRAs and CD8 T cells but did not observe a significant reduction in the HIV infectious reservoir. Using both ex-vivo and in-vivo mouse models, they demonstrated that lack of reduction of the infectious reservoir was not due to immune escape and inability of CD8 T cells to recognise infected T cells or lack of activation by LRAs to induce proliferation of quiescent CD4 T cells.
Studies of HIV proviruses in CD4 T cells from ART treated individuals, have demonstrated a role provirus type in viral replication, where intact inducible provirus are associated with the lower viral replication compared to defective provirus, one that has insertion and deletions. In fact previous data by the by the research group demonstrated that a subset of defective proviruses are associated with increased recognition by CD8 T cells.
In summary, data presented by Huang et al., suggests that inability of LRAs and CD8 T cells to reduce to the HIV infectious reservoir could be due to the presence of intact-inducible virus that prevents CD8 T cell mediated elimination due to lack of expression of viral antigens. The exact nature of this mechanism requires further research.
Journal Article: Huang et al., 2018. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. Journal of Clinical Investigation
Article by Cheleka AM Mpande