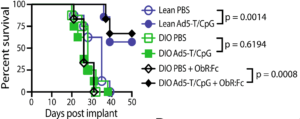
Figure 4B Murphy et al., :Lean and DIO mice were implanted intrarenally
with Renca (2 3 105 cells), followed by PBS or Ad5-TRAIL/CpG (Ad5-T/CpG) treatment on day 7. Some DIO mice received ObR:Fc on day 2–4 (100 mg i.v.daily) to neutralise leptin.
Obesity is associated with increased risk of developing cancer, as well as cancer-associated morbidity and mortality. Current cancer immunotherapies for obese individuals have positive effects in murine models, however limited efficacy is observed in humans. A suggested reason for this disparity is the use of “inappropriate models” during pre-clinical stages.
Murphy et al., investigated the impact of obesity on immunotherapuetic strategies. The two therapies tested were the (i) experimental intratumoural delivery of recombinant adenovirus encoding TRAIL in combination with TLR agonist CpG (Ad5-T/CpG) and (ii) systemic administration of checkpoint inhibitor (anti-CTLA-4 monoclonal antibody). These therapies were tested on lean (healthy), leptin-deficient and diet-induced obese (DIO) mice. Leptin is a hormone secreted by adipocytes, in the absence of leptin satiation is not achieved which leads to excessive eating and eventually obesity. In DIO mice increasing adiposity results in increased leptin which can lead to leptin resistance and ultimately overeating. Obesity in humans is also associated with above normal levels of leptin, thus studying the effect of immunotherapies in DOI mice, is one of the best clinically relevant murine models.
Researchers showed that immunotherapeutic interventions described above were successful in healthy and leptin-deficient mice but not in DIO mice. Immune phenotyping of the mice showed that DIO mice have functionality impaired dendritic cells (DC) determined by lower expression of CD86, CD80 and CD40. Impairment of DC function was associated with lower tumour infiltration by CD8 T cells in DOI mice, suggesting that reduced efficacy of Ad5-T/CpG and anti-CTLA-4-mAb could be due to insufficient priming of CD8 T cells.
Finally, Murphy et al., demonstrated that reduced efficacy of immunotherapy is due to the inhibitory effect of excessive leptin. Where neutralisation of leptin activity by blocking the leptin receptor, restores therapeutic efficacy of the treatment determined by increased survival, as well as higher DC expression of CD86 and increased CD8 tumour infiltration compared to untreated DOI mice.
In summary, Murphy et al., showed the utility of leptin neutralisation in improving cancer immunotherapy in diet induced obesity.
Article: Murphy et al., 2018. Cutting Edge: Elevated Leptin during Diet-Induced Obesity Reduces the Efficacy of Tumour Immunotherapy. Journal of Immunology.
Article by Cheleka AM Mpande










