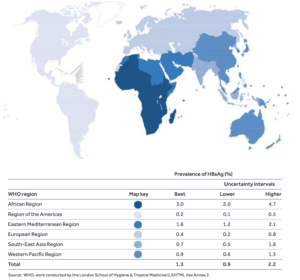
Hepatitis B (HBV) remains a global health problem, with two billion people having serologic evidence of past or ongoing HBV infection.An estimated 257 million people, 3.5% of the global population, are chronically infected with HBV. Although HBV and its associated complications of cirrhosis, liver failure and hepatocellular carcinoma are entirely vaccine preventable, there is no cure for chronic hepatitis B as yet. More so, the wide spread of multidrug-resistant HBV strains is increasingly threatening the efficacy of currently available antiviral drugs hence, the urgent need to develop and test promising novel therapies to target HBV.
Previous studies have considered Ad-HBV as primary HBV immunotherapeutic agent, with the aim of application for treating patients chronically infected by HBV and Pharmacodynamics studies in three animal models have been done thus far (HLA-A2 transgenic mice, C57BL6 mice, and BALB/c mice). Due to the damage to the host immune response, hepatocytoxicity, and a short half-life observed in these studies clinical development and trial as a vaccine has not been done.
Researchers at the Nanjing Medical University and Tripod Preclinical Research Laboratories in China have assessed the immunogenicity of Ad-HBV administered to cynomolgus monkeys as non-human primate model, by monitoring the induced HBV-specific T cell responses in a three-month repeat dose toxicity study (a non-clinical safety assessment) with the aim of determining its suitability for use in clinical trials as an anti-HBV infection vaccine.
Zhang et al., observed that there was no serious toxicity problem. Overall, their findings demonstrated that the main toxicity of Ad-HBV is related to stimulation at the injection site by the Ad itself, which resulted in erythema on the injection point skin. The transient increase also observed in peripheral blood lymphocyte subset were still within normal ranges, so they did not have toxicological significance. Differences observed in some T cell subsets in the different doses also had no biological significance since it was change in a single index and was not dosing depended.
From the data generated by the researchers from the non-clinical safety assessment, Ad-HBV should be selected as a lead candidate for clinical development since the Ad-HBV immunotherapeutic product induced no biologically significant toxicity, it induced strong and broad IFNγ+ cell responses, and displayed a whole array of immunogenic characteristics, demonstrating its high immunogenic potential.
Journal Article: Zhang et al., 2018. Immunogenicity of adenovirus-vector vaccine targeting hepatitis B virus: non-clinical safety assessment in non-human primates. Virology Journal.
Article by Margaret Adeoti