Macrophages are the first line of defense (innate immunity). Depending on anatomical location tissue resident macrophages differentiate into specialized macrophages. Majority of tissue resident macrophages are established before birth and maintain their populations by self-renewal. Studies have shown that bone derived monocytes are able to infiltrate tissue and differentiate in to specialized tissue macrophages.
Macrophage disappearance reaction is the depletion of macrophages due inflammation, this provides a niche for infiltration monocytes to replenish the compartment. Necroptosis is a type of macrophage depletion reaction that is mediated by bacterial infection. Infection mediated necroptosis has not been characterized in response to non-bacterial infection. Study by Lai et al., aimed to determine the impact of non-bacterial (malaria) infection on tissue resident macrophage disappearance.
Lai et al., utilized a murine model of blood stage malaria, where mice are infected with non-lethal Plasmodium yoelii which self resolves. Blood stage malaria causes a systemic infection and affects multiple organs such as lungs, spleen and liver. Additionally, CD169+ macrophages play a crucial role in anti-malarial responses limiting infection-induced inflammation, however they also contribute to severe pathology. This makes using such a model ideal to test macrophage disappearance reaction due to a parasite.
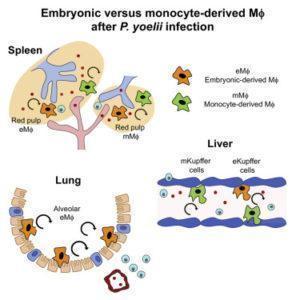
Kupffer cells (liver resident macrophages) are well positioned to be first line responders to the liver stage of malaria infection. By 3 days post malaria infection, in the presence of low level parasitemia researchers observed a significant disappearance of Kupffer cells, as well as a significant recruitment of inflammatory monocytes which differentiated to activated macrophages. After parasite clearance these bone-marrow derived macrophages adopted phenotypic and functional characteristics of Kupffer cells, with a similar turnover rate. They observed similar kinetics of red pulp macrophages (spleen resident macrophages). Interestingly, they also observed a decrease of alveolar (lung) macrophages during parasitemia, followed by a steady increase of these macrophages post parasite clearance. This increase in the macrophage population was due to self-renewal of the remaining alveolar macrophage and not due to recently infiltrated monocytes. This demonstrates that unlike in the liver and spleen, the alveolar macrophage compartment is a closed system with no contribution from bone marrow derived monocytes.
In summary, this study shows the dynamic and tissue specific nature of tissue resident macrophages during a plasmodium infection. Illustrating that some tissue resident macrophage compartment but not all are maintained by a contribution of bone derived monocyte during infection.
Journal Article: Lai et al., 2018. Organ-Specific Fate, Recruitment, and Refilling Dynamics of Tissue-Resident Macrophages during Blood-Stage Malaria. Cell Reports
Article by Cheleka AM Mpande