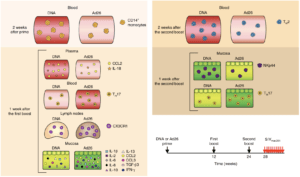
Figure 6 Vaccari et al., Schematic of correlates of protection and the risk of acquisitions measured after inoculation of prime or each of the boosts in different compartments. TGF-β3, transforming growth factor-β3. Arrows represent time of vaccination (black, weeks 0–24) or challenges (red, week 28).
The RV144 vaccine trial is the only HIV vaccine trial that has shown limited but significant efficacy of 31.2%. The trial showed that vaccination with HIV recombinant Aventis Pasteur’s canarypox vector (ALVAC-HIV) and AIDSVAX B/E (gp120-vaccine), prime-boost, induced high levels of HIV-envelope (Env)-specific CD4 T cell and antibody responses, but negligible CD8 T cell responses. A similar efficacy trial of an SIV*-based vaccine regiment showed decreased risk of SIV acquisition, with an efficacy of 44% in macaques. Vaccari et al., hypothesized that changing vaccine type and/or vector may improve efficacy of the ALVAC-SIV + gp120 alum vaccine. They tested a DNA-SIV vaccine (containing DNA constructs of SIV P57gag, P39faf, gp120 and LAMP-Pol genes) that was hypothesised to increase CD4 T cells responses, as well as Ad26-SIV (adenovirus serotype 26 containing SIV Gag, Pol and Env proteins) hypothesised to increase mucosal antibody and CD8 T cell responses
Researchers vaccinated macaques with two intramascular doses of DNA-SIV, one dose of Ad26-SIV or two doses of ALVAC-SIV, this was followed by two doses of ALVAC-SIV/gp120 alum vaccine boosts. Researchers showed that DNA-SIV and ALVAC-SIV but not Ad26-SIV were effective at decreasing risk of SIV acquisition in the macaques. Vaccari et al., observed differences in innate and adaptive immune responses between macaques primed with DNA-SIV and Ad26-SIV. Where Ad26-SIV prime resulted in enhanced differentiation of monocytes, increased levels of cytokines at mucosal sites and increased levels of long-lasting mucosal Th17 cells compared with DNA-SIV vaccination. Decreased risk of SIV acquisition in DNA-SIV primed macaques was associated with activation of the hypoxia and inflammasome pathways in classical (CD14+CD16-) monocytes as well as increased levels of CCR5- Th2 cells and cyclic V2 antibodies.
Based on this study, researchers showed that monocyte innate immunity primed by a DNA-vaccine contributed decreased risk of SIV acquisition either by causing an inflammatory environment or indirectly influences cellular and humoral adaptive responses. The exact mechanism that results in reduced viral acquisition is still be determined.
*SIV: Simian Immunodeficiency Virus.
Journal Article: Vaccari et al., 2018. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14+ monocytes is associated with a decreased risk of SIVmac251 acquisition. Nature Medicine
Article by Cheleka AM Mpande











