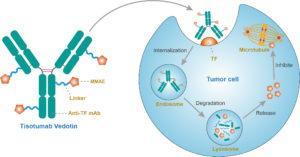
Mechanism of action of tisotumab vedotin (Source: https://www.creativebiolabs.net/tisotumab-vedotin-overview.htm)
Tissue factor is expressed across multiple solid tumour types and is associated with poor clinical outcomes. Studies have shown that it can contribute to cancer pathology by promoting metastasis, tumour growth, and tumour angiogenesis, hence suggesting it as probable target for therapeutic intervention.
Prior to the study by Professor De Bono and colleagues, no previous study had considered the use of Tisotumab Vedotin, a tissue factor-specific human monoclonal antibody as a treatment option for patients with cancer. The group selected Tisotumab Vedotin from a panel of tissue factor-specific human monoclonal antibodies due to its high affinity binding to tissue factor and its capacity for interfering with protease activated receptor 2 intracellular signalling, as assessed by inhibition of tissue factor: FVIIa-dependent ERK phosphorylation and interleukin-8 production in tissue factor-positive tumour cells.
The researchers carried out a Phase 1/2 clinical trial, enrolling patients with various cancer types, (including ovary, cervix, endometrium, bladder, prostate, oesophagus, non-small-cell lung cancer, or squamous cell carcinoma of the head and neck) known to express tissue factor and are susceptible to microtubule-disrupting agents. Eight cohorts of tisotumab vedotin, ranging from 0∙3 to 2∙2 mg/kg was administered intravenously once every 3 weeks in the dose escalation phase. Dose-limiting toxicity observed in the 27 patients enrolled in this phase 1 trial led to a choice of 2∙0 mg/kg as the recommended phase 2 dose, which was the dose-expansion phase which included 147 cancer patients.
De Bono et al., 2019 showed that 15∙6% (95% CI 10∙2–22∙5; 23 of 147 patients) achieved an objective response across different tumour types but also observed numerous treatment-emergent adverse events ranging from epistaxis, fatigue, nausea, alopecia, conjunctivitis, decreased appetite, constipation, diarrhoea, vomiting, peripheral neuropathy, dry eye, and abdominal pains. Nine deaths were also observed across all study phases (three in the dose escalation phase and six in the dose-expansion phase.
Tisotumab vedotin had an encouraging antitumor activity across multiple tumour types as well as a manageable safety profile which raises hope of this drug as a good candidate for cancer treatment in patients known to express tissue factor. However, continued evaluation for safety and tolerability is needed.
Journal Article: De Bono et al., 2019. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1–2 trial. Lancet Oncology
See also: Tisotumab Vedotin (HuMax®-TF-ADC) Safety Study in Patients With Solid Tumors
Article by Margaret Oluwatoyin Japhet











