In a recent study from the preprint server, bioRxiv, researchers have demonstrated that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD)-based, sortase A-conjugated nanoparticle (RBD-scNP) vaccine induces neutralizing antibodies (NAbs) in non-human primates (NHPs) against all eight variants of SARS-CoV-2 (Read more: Using nanoparticles in HIV immunogen design, Is a self-amplifying RNA SARS-CoV-2 lipid nanoparticle a good vaccine candidate?).
Using RBD-scNPs vaccine formulations to immunise cynomolgus macaques, the results were then analysed to assess the neutralizing activity of sera against pseudovirus infection of 293T-ACE2-TMPRSS2 cells by SARS-CoV-2 WA-1 to WA-8 strains (Figure 1). They also investigated whether the SARS-CoV-2 Omicron variant could evade the RBD-scNP-induced Nabs. Additionally, the authors also provided insight into dosing strategies using RBD-scNPs.
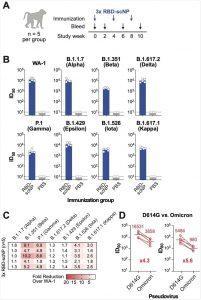
Figure 1. RBD-scNP vaccination elicits broad neutralizing antibodies against SARS-CoV-2
variants. (A) Schematic of the vaccination study in macaques. Cynomolgus macaques (n = 5 per group) were immunized intramuscularly 3 times with 100 μg of RBD-scNP adjuvanted with 3M-052-AF + Alum. The control group was immunized with PBS. Blood and mucosal samples, including Bronchoalveolar lavage (BAL) and nasal swab samples, were collected in the indicated time points for antibody assays. (B-C) Plasma antibody (post-3rd immunization) neutralization of SARS-CoV-2 variants pseudovirus infection of 293T-ACE2-TMPRSS2 cells. (B) Neutralization titers were shown as 50% inhibitory dilution (ID50). (C) Reduction of neutralization titers against variants were shown as fold reduction compared to the titers against WA-1. (D) Plasma antibody (post-3rd immunization) neutralization titers against pseudoviruses of the SARSCoV-2 Omicron variants in 293T-ACE2 cells. The mean ID50 and ID80 titers and the fold reduction compared to D614G are shown (Li, D., et al., 2022).
Previously, Li, et al., have reported on a RBD-scNP vaccine formulation with the 3M-052-aqueous formulation (3M-052-AF) plus Alum that elicited cross-reactive NAbs against SARS-CoV-2 and other sarbecoviruses and conferred protection against the SARS-CoV-2 WA-1 strain in NHPs. Additionally, they found that RBD-scNPs induced antibodies that neutralized all SARS-CoV-2 variants.
In their own words:
“our study demonstrates that scNP vaccines with SARS-CoV-2 spike or spike subunits confer 339 potent protection in NHPs against WA-1, Beta and Delta variants, and that they induce neutralizing 340 antibodies to all SARS-CoV-2 variants tested in vitro. These findings have important implications for 341 development of the next generation of COVID-19 vaccines.”
It must be noted that the study did have several limitations.
NB to note: bioRxiv is a preprint server which publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, or guide clinical practice or treated as established information.
Journal article: Li, D., et al., 2022. Breadth of SARS-CoV-2 Neutralization and Protection Induced by a Nanoparticle Vaccine. bioRxiv.
Summary by Stefan Botha










