For decades, developing a safe and effective HIV vaccine has been a critical scientific goal. One major hurdle is the virus’s rapid mutation rate, creating a vast array of strains within a single infected person. However, recent research by a collaborative team offers promising new insights into an immunization strategy that could overcome this challenge.
New research represents a significant step forward in an immunization approach for HIV, that targets the body’s production of broadly neutralizing antibodies (bnAbs) (Figure 1).
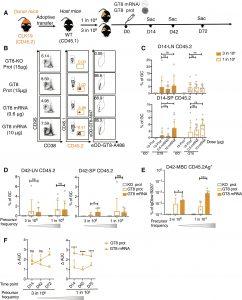
Figure 1: CLK19 B cell responses induced by eOD-GT8 60mer mRNA-LNP. (A) Schematic: Eight-week-old wild-type (WT) CD45.1 mice adoptively transferred with B cells from CLK19 CD45.2 mice to establish precursor frequencies of ~1 in 104 or 3 in 106 B cells. Mice were then immunized with eOD-GT8 60mer formulated with alum (15 μg), a CD4bs-KO control (eOD-GT8–KO 60mer, 15 μg), or eOD-GT8 60mer mRNA-LNP (0.6 or 10 μg) 1 day after transfer (D0). Splenocytes or lymph nodes (LNs) were isolated on D14, D42, and D72. “mRNA” is used to mean “mRNA-LNP” throughout, and “prot” stands for “protein.” (B) Flow cytometry of D14 LNs from mice (1 in 104) as per (A). CD45.2-binder populations gated as Scatter/Singlet/Live (SSL+)/B220+/CD95+CD38−/CD45.2+/Ag+. GC percentage (first column), CD45.2 in GCs (second), and Ag+ CD45.2 (third). (C) D14 LN (top) or spleen (bottom) quantification. The x axis represents immunization groups, and the y axis represents the percent CD45.2 within GCs. Circles represent one mouse. Two independent experiments were pooled for analysis. n = 3 to 5 mice per independent group. (D) D42 quantification. The x axis indicates treatment group, and the y axis represents the percentage of CD45.2 within GCs. Two independent experiments were pooled for analysis. n = 3 or 4 mice per independent group. (E) Ag+ CD45.2 MBC frequencies from D42 splenocytes. The gating strategy is shown in fig. S1E. x axis shows immunization group precursor frequencies, and y axis represents the percentage of Ag+ CD45.2 MBCs among the B220+IgDlo GC population and starts at 0.0001. Two independent experiments were pooled for analysis; n = 3 or 4 mice per independent group. Circles represent individual mice. (F) IgG titers from sera after immunization by eOD-GT8 60mer protein (15 μg) or mRNA-LNP (10 μg). n = 3 to 10 mice in each group from two independent experiments. The x axis indicates the day, and the y axis indicates the change of area under curve (AUCcoated eOD-GT8 − AUCcoated eOD-GT8 KO). Where shown, bars indicate geometric means and geometric SD of pooled groups, and significance was calculated with Student’s t test (D to F) or one-way ANOVA (C): P > 0.05 represents no significance (ns), *P < 0.05, ***P < 0.001, and ****P < 0.0001.
BnAbs are the immune system’s elite soldiers against HIV, capable of blocking a wide range of viral variants. Unfortunately, the human body naturally produces very few bnAbs in response to HIV infection. The IAVI trial focused on stimulating the immune system to generate the specific precursor B cells that can eventually evolve into these coveted bnAbs.
The strategy involves a two-step process: priming and boosting. The priming step utilizes a customized molecule, called an immunogen, to activate the correct B cell precursors. However, these precursors require further development towards bnAbs. This is where booster immunogens come in. These additional immunogens, some utilizing mRNA technology, further guide the primed B cells along the path towards becoming mature bnAbs.
Four studies explored different aspects of this approach. One successfully primed exceptionally rare BG18 precursors in animal models. Subsequent structural analysis confirmed the development of the desired BG18-class antibodies. This finding suggests a high probability of success in human trials.
The second, built upon the priming concept. Here, they used a combination of priming and RNA-based boosting to nudge the primed B cells towards recognizing more natural forms of HIV. This demonstrates the feasibility of guiding B cell development towards bnAbs. The third study focused on VRC01-class bnAbs, another potent weapon against HIV.
The final study, delved deeper into the immunological processes within germinal centers, specialized structures where B cells refine their antibody response.
Collectively, these studies offer compelling evidence for the effectiveness of the priming step in activating the desired bnAb precursors. Moreover, three of the studies demonstrate the possibility of guiding these precursors towards becoming mature bnAbs capable of fighting HIV infection.
Journal article: Wang, X., et al. 2024. mRNA-LNP prime boost evolves precursors toward VRC01-like broadly neutralizing antibodies in preclinical humanized mouse models. Science Immunology.
Summary by Stefan Botha










