Glioblastoma, one of the most aggressive and treatment-resistant brain cancers, may soon have a new weakness. Despite its prevalence, survival rates have barely improved over the past two decades. Current treatments—surgery, radiation, and chemotherapy—offer limited benefits. Even immune checkpoint inhibitors, which have revolutionized treatment for many other cancers, haven’t made a dent in glioblastoma outcomes.
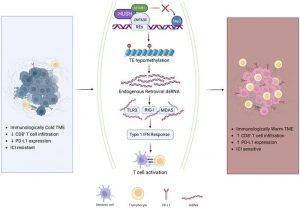
Scientists have discovered that suppressing a protein called ZNF638 can trigger an antiviral immune response in glioblastoma tumours, making them more vulnerable to immune checkpoint inhibitors (ICIs) (Figure 1). This breakthrough could finally bring immunotherapy within reach for patients suffering from this deadly cancer.
Over millions of years, humans have accumulated fragments of viral DNA—known as human endogenous retroviruses (HERVs)—within their genomes. Normally, our cells keep these fragments silent with the help of a protein complex called HUSH, regulated by ZNF638. But when ZNF638 is suppressed, those ancient viral fragments wake up—not enough to cause infection, but enough to trigger a powerful antiviral immune response. The immune system floods the tumour with immune cells, creating an opportunity for ICIs to work.
Viral mimicry has already shown promise in cancers like ovarian cancer since 2015. But this is the first time it has demonstrated potential in brain tumours. The team began by analysing patient genetic data and found that glioblastoma patients with naturally lower ZNF638 expression had better responses to immune checkpoint therapy and longer survival rates. Tumours with reduced ZNF638 also had more immune cell infiltration—a clear sign that viral mimicry was at play.
Next, the team conducted preclinical studies, suppressing ZNF638 in glioblastoma tumour cells in animal models. The results were:
- Tumor growth slowed
- T-cell infiltration increased
- Survival times improved significantly
One of the most exciting aspects of the discovery is the potential to use ZNF638 as a biomarker. Biomarkers help identify which patients are most likely to benefit from specific therapies—in this case, immune checkpoint inhibitors. Currently, ICI therapy for glioblastoma is highly experimental and not approved by the FDA. But using ZNF638 expression as a guide could make personalized immunotherapy a reality for glioblastoma patients.
For glioblastoma patients, this discovery offers hope at last. While immunotherapy has transformed the treatment landscape for cancers like melanoma and lung cancer, glioblastoma has remained a notoriously tough opponent.
Journal article: Seetharam, D., et al, 2025. Activating antiviral immune responses potentiates immune checkpoint inhibition in glioblastoma models, Journal of Clinical Investigation.
Summary by Stefan Botha