Up to 59 000 people die from rabies virus infection each year. The victims often don’t know if they have been exposed to the virus. In addition, the treatment cost for rabies is expensive and some cannot afford this. Vaccination against rabies is both a cheaper and, in the long term, better option in terms of both prevention and safety (READ MORE).
With the current rabies vaccines on the market, they do not provide lifelong protection, with the need for booster vaccine shot every odd year. Currently rabies vaccines come from killed viruses whereby, in some cases, the immunity inducing molecules can change shape and no longer provide the immune responses with the correct recognition molecule.
In a recent paper, Callaway, et al., identified and described a potential new and improved rabies vaccine design (Figure 1). They described a high-resolution structure of the rabies virus glycoprotein in a trimeric form. This protein is expressed on the surface of the virus, making it an ideal target for vaccine design.
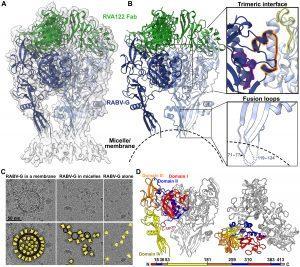
Figure 1: Structure of the prefusion RABV-G trimer bound to neutralizing antibody RVA122. (A) Molecular model of RABV-G (blue) and RVA122 Fab (green) complex fitted into the corresponding cryo-EM density map. (B) RABV-G trimer with magnified views of the trimeric interface (top right) and fusion loops (bottom right). In the trimeric interface, the small helix (purple), bracket loop (orange), and corkscrew loop (yellow) are highlighted. (C) Top: Raw micrographs of glycoprotein complexes embedded in cellular membranes (left), embedded in micelles (center), or unembedded (right). Bottom: RABV-G (yellow), membranes (black, double outline), and micelles (black, single outline) are indicated. (D) Side view (left) and top view (right) of the RABV-G trimer, with domains I to IV and the C terminus indicated for one protomer. Colors correspond to RABV-G domains I to IV, also shown in the schematic diagram of the RABV-G sequence (Callaway, et al., 2022).
Researchers think that proteins expressed by the rabies virus mutate or change in structure which give it the ability to escape immune recognition. The researchers in this study were able to stablise the glycoprotein, in a pre-fusion form which is the shape that it takes before infection of cells. This image was capture in 3D using cryo-electron microscopy and the researchers were able to identify the antibody which attaches to the glycoprotein, marking it for attack by the immune system.
They hope that information provided about this structure will lead to improved vaccine design and development against the rabies virus. This is in turn will lead to improved immunity which can last much longer.
Journal article: H.M. Callaway, H.M., et al., 2022. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science Advances.
Summary by Stefan Botha










