Currently, diagnosis of SARS-CoV-2 infection, the etiological agent of coronavirus disease 2019 (COVID-19), is predominantly based on molecular detection of SARS-CoV-2 viral genetic material. An alternative method for diagnosing SARS-CoV-2 infection would be using serological tests that detect SARS-CoV-2-specific antibody (Ab) responses, similar to the Alere™ HIV Combo – Rapid Test. However, no SARS-CoV-2 serological assays are available at point of care nor have been recommended by the World Health organisation (WHO).
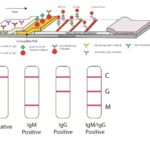
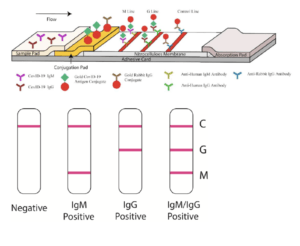
Guo et al., were among the first to describe Ab kinetics during COVID-19, they showed that SARS-CoV-2-specific IgM and IgA Ab responses are detectable within the first 5 days of infection (symptom presentation), whereas SARS-CoV-2-specific-IgG responses are detectable during later phases of infection (>10 days). In line with these results, Li et al., described the development of a “rapid lateral flow immunoassay which can detect IgM and IgG Ab simultaneously against SARS-CoV-2 virus in human blood within 15 minutes.” In classical Ab immunity, IgM Abs are induced early upon infection before IgG Abs. As a result, these test can theoretically can detect immune responses induced early upon infection, as well as upon COVID-19 recovery (IgG immunity). Pre-print by Amanat et al., (Now Published in Nature Medicine) also described the development of a serological enzyme-linked immunosorbent assay (ELISA) developed using recombinant antigens derived from the spike protein of SARS-CoV-2. The assay was used to test blood samples from COVID-19 patients alongside negative control samples representing pre-COVID-19 background immunity in the general population.
Serological assays could be useful for identifying highly reactive potential human donors (who generate convalescent sera) and for screening of health care workers to identify those who are already immune to the virus (minimising the risk of viral spread when caring for infected patients). Additionally, they can be used to rule out SARS-CoV-2 infection in individuals that are negative for molecular detection of SARS-CoV-2.
References:
- Amanat et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nature Medicine.
- FIND Evaluation Update: Sars-Cov-2 Immunoassays
- Guo et al., 2020. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clinical Infectious Disease
- Li Z et al. 2020. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. Journal of Medical Virology
- Petherick. 2020. Developing antibody tests for SARS-CoV-2. The Lancet.
- Okba et al., SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv Pre-print.
- WHO Scientific Report: Advice on the use of point-of-care immunodiagnostic tests for COVID-19.
Article by: Sawsan Feki and Cheleka AM Mpande