Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes joint damage and reduces quality of life. Most RA treatments focus on managing symptoms after the onset of the disease. However, a ground-breaking study published in The Lancet (2024) offers new hope for preventing RA in individuals at high risk of developing the condition. The study, titled “Abatacept Inhibits Inflammation and Onset of Rheumatoid Arthritis in Individuals at High Risk (ARIAA),” evaluates the efficacy of abatacept, a T-cell inhibitor, in reducing the risk of RA in people with preclinical signs of the disease.
Further Reading: Precision Medicine Tool Offers Hope for Tailored Rheumatoid Arthritis Treatments
Study Design and Methods
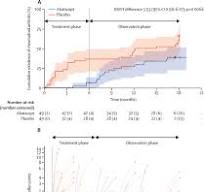
The ARIAA study was a randomized, double-blind, placebo-controlled trial conducted across 14 centres in Europe. Participants included 98 adults with anti-citrullinated protein antibodies (ACPAs), joint pain, but no visible swelling—making them highly susceptible to developing RA. The participants were divided into two groups: one received abatacept, a T-cell inhibitor, while the other received a placebo. Both treatments were administered for six months, followed by a drug-free observation period of 12 months.
The primary outcome was the reduction of subclinical inflammation, measured via MRI. Subclinical inflammation, often present before RA symptoms manifest, includes early joint changes like synovitis and tenosynovitis, detectable by MRI.
Key Results
The results were promising. After six months, 57% of the participants treated with abatacept showed a significant reduction in MRI-detected inflammation, compared to only 31% in the placebo group. Even more impressive was the fact that only 8% of the abatacept group progressed to full-blown RA, compared to 35% in the placebo group.
These results held up even after 18 months, with abatacept continuing to reduce the progression of RA, despite the absence of treatment during the observation phase. This suggests that abatacept may not only reduce inflammation but also potentially modify the disease course.
Safety and Side Effects
The safety profile of abatacept was consistent with previous studies. Serious adverse events occurred in 8% of the abatacept group and 14% of the placebo group. No deaths were reported, and the overall safety data supported the use of abatacept in individuals at high risk for RA.
Implications for RA Prevention
The ARIAA study’s findings represent a major breakthrough in RA treatment strategies. Abatacept’s ability to halt the progression of RA in its early, preclinical stages could change how the disease is managed. Currently, RA treatments focus on controlling inflammation after the disease has fully developed. This study opens the possibility of using abatacept to prevent RA altogether in high-risk populations.
By targeting T-cell activation, abatacept addresses one of the key drivers of RA. The success of this approach could lead to further research on using similar therapies to prevent other autoimmune diseases.
The ARIAA study demonstrates that abatacept is a promising treatment for preventing the onset of RA in individuals at high risk. Its ability to reduce subclinical inflammation and prevent disease progression offers a new, proactive approach to managing RA. Further studies will help determine the long-term benefits of this treatment and its potential use in broader populations.
Journal Article: Rech, Juergen, et al. “Abatacept Inhibits Inflammation and Onset of Rheumatoid Arthritis in Individuals at High Risk (ARIAA): A Randomised, International, Multicentre, Double-Blind, Placebo-Controlled Trial.” The Lancet.
Summary by Faith Oluwamakinde