Acute kidney injury (AKI) remains a significant clinical challenge with a poor prognosis. Despite extensive research, effective therapeutic interventions remain elusive. A key to understanding AKI’s progression lies in the complex interplay of immune cells and their signaling molecules.
Macrophages, immune cells crucial for tissue repair and inflammation, play a pivotal role in AKI. They produce lipid mediators (LMs), bioactive lipids that can both promote and suppress inflammation. Understanding how LMs influence AKI’s course is essential for developing targeted therapies.
Recent research has shed light on the role of the transcription factor MAFB in regulating macrophage function during AKI (Figure 1). MAFB is known to influence the inflammation-suppressive properties of these cells.
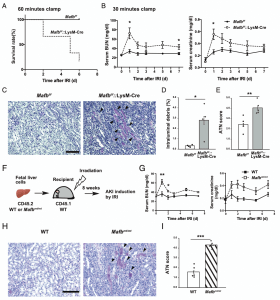
Figure 1: Loss of Mafb in macrophages worsens the prognosis of ischemic AKI. (A) Survival curve of Mafbf/f and Mafbf/f::LysM-Cre mice subjected to IRI with 60-min clamp (n 5 6 for each group). p < 0.01, by log-rank test. (B) Serum BUN and creatinine levels of Mafbf/f and Mafbf/f::LysM-Cre mice subjected to IRI with 30-min clamp (n 5 6 for Mafbf/f, n 5 5 for Mafbf/f::LysM-Cre). (C) Representative periodic acidŸSchiff (PAS)Ÿstained kidney from Mafbf/f and Mafbf/f::LysM-Cre mice at 7 d post-IRI. Scale bar, 100 mm. Arrowheads point to intraluminal debris. (D and E) Quantification of PAS1 intraluminal debris at the corticomedullary junction (D), as well as the acute tubular necrosis (ATN) scores (E) in the kidneys of Mafbf/f and Mafbf/f::LysM-Cre mice at 7 d post-IRI. Intraluminal debris (D) is presented as a percentage area per whole corticomedullary junction in a slide. (F) Fetal liver cells of WT or MAFBp.Leu239Pro (Mafbmt/mt) CD45.2 embryo were transplanted to lethally irradiated WT CD45.1 mice. After the 8-wk recovery period, mice were subjected to renal IRI. (G) Serum blood urea nitrogen (BUN) and creatinine levels of WT or Mafbmt/mt cellŸtransplanted mice subjected to IRI with a 30-min clamp. n 5 5 for each group. (H) Representative PAS stained kidney from WT or Mafbmt/mt cellŸtransplanted mice at 7 d post-IRI. Scale bar, 100 mm. Arrowheads point to intraluminal debris. (I) The ATN scores in the kidneys of WT or Mafbmt/mt cellŸtransplanted mice at 7 d post-IRI. All data are expressed as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, by Welch t test
In mice lacking MAFB specifically in macrophages (MAFB-deficient mice), AKI was more severe compared to wild-type mice. Further analysis revealed that MAFB deficiency led to a significant reduction in the expression of ALOX15, an enzyme essential for the production of pro-resolving LMs.
Interestingly, the pro-inflammatory lipid mediator PGE2 was found to regulate MAFB expression in macrophages during AKI. Moreover, MAFB itself influenced ALOX15 expression under the influence of PGE2. These findings suggest a critical role for MAFB in shifting the balance of LMs from pro-inflammatory to pro-resolving states.
Given that the balance between pro-inflammatory and pro-resolving LMs significantly impacts the outcome of acute inflammation, these findings have important implications for the development of novel therapeutic and diagnostic approaches for AKI and other inflammatory diseases. By targeting MAFB or its downstream pathways, researchers may be able to modulate macrophage function and improve patient outcomes.
Journal article: Kanai, M., et al., 2024. MAFB in Macrophages Regulates Prostaglandin E2–Mediated Lipid Mediator Class Switch through ALOX15 in Ischemic Acute Kidney Injury, The Journal of Immunology.
Summary by Stefan Botha










