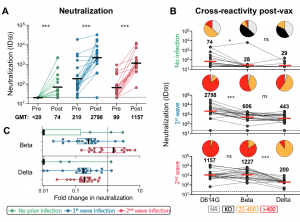
Neutralizing antibody responses to Ad26.COV2.S vaccination after prior infection. A. Neutralization of the SARS-CoV-2 D614G pseudovirus by plasma pre- and post-vaccination from participants with no prior infection (green, n=19) and those infected in the first (blue, n=20) and second waves (red, n=19). Neutralization is reflected as an ID50 titer. The threshold for positivity is indicated by a dotted line B. Cross-reactive neutralization post-vaccination against D614G, Beta and Delta variants. Pie charts show the proportion of vaccine non-responders (NR; grey), knock-out of neutralization of Beta or Delta (KO; black), titer of 20-400 (orange), or >400 (red). C. Fold change of post-vaccination D614G neutralization titers relative to Beta or Delta. The dotted line indicates a fold change of 1 (no change). The horizontal black and red bars indicate geometric mean titers, with values indicated on the graphs. Statistical analyses were performed using the Mann-Whitney test between groups, and the Wilcoxon test for paired analyses. * denotes p<0.05, *** p<0.001, ns, non significant. Experiments were performed in duplicate with the average value shown. (Source: Keeton et al., Pre-print)
COVID-19 vaccines are provided to all individuals, regardless of previous SARS-CoV-2 infection. We have previously highlighted research findings that showed vaccination with BNT162b2 (Pfizer/BioNTech) significantly boosts pre-existing SARS-CoV-2 immunity induced by natural infection (Read more: Antibody response to vaccination post-COVID-19 infection). Is this boosting effect observed in individuals vaccinated with other COVID-19 vaccines?
Ad26.COV2.S vaccine, single-dose adenovirus 26 vectored vaccine expressing the SARS-CoV-2 Wuhan-1 stabilized spike (S) protein, is highly effective against the development of severe COVID-19 was the first vaccine to be rolled in South Africa. The single-dose regimen makes it one of the most ideal vaccines to facilitate rapid roll-out in Africa. In a recent Pre-print, Keeton et al., “examined the effect of prior infection with ancestral (D614G) or Beta variants on Ad26.COV2.S immunogenicity approximately 28 days post-vaccination.”
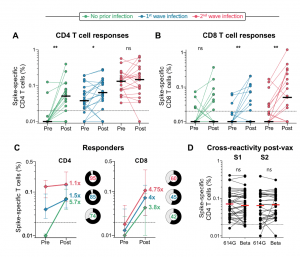
T cell responses to Ad26.COV2.S vaccination in the context of prior infection. A. Frequency of total cytokine-producing spike-specific CD4 T cells, and B. CD8 T cells, in those with no prior infection (green, n=19), infection in the first wave (blue, n=20), and infection in the second wave (red, n=19), in PBMC stimulated with a peptide pool based on Wuhan spike. C. Median fold-change of CD4 and CD8 T cell frequencies after vaccination in responders. Error bars indicate interquartile range. Pie charts indicate the proportion of responders (black) and non-responders (grey), indicated in the centre. D. Cross-reactivity of T cell responses post-vaccination. The frequency of cytokine-producing CD4 T cells post-vaccination in response to peptide stimulation with the S1 or S2 regions of spike from D614G or Beta is shown. Horizontal black or red bars indicate medians. The dotted line indicates the threshold for positivity and values are background-subtracted. Statistical analyses were performed using the Wilcoxon test. * denotes p<0.05, ** p<0.01, ns = non-significant. (Source: Keeton et al., Pre-print)
Researchers showed that Ad26.COV2.S vaccination boosted circulating S-protein specific antibodies (Abs), neutralizing Abs (nAbs) and antibody-dependent cellular cytotoxicity (ADCC) in individuals previously infected during the 1st and 2nd waves to levels much higher than naïve individuals. Surprisingly, despite the longer time interval between wave 1 infection and vaccination (7 months), compared to wave 2 (2 months) the overall magnitude of vaccine-induced boosting was similar. Interestingly, researchers observed no substantial vaccine-induced boosting of S-specific CD4 T cells responses in previous SARS-CoV-2 infected individuals.
In summary, “Ad26.COV2.S vaccination following prior infection, even >6 months previously, may result in substantially enhanced protection against COVID-19, of particular relevance in settings of high SARS-CoV-2 seroprevalence.” These results, also highlight the potential role of cellular immunity in maintaining long-lasting immunity against future variants as results suggest that T cell recognition is not largely affected by mutations present in variants of interest.
Journal Article: Keeton et al., 2021 (Pre-print). Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant dependent manner. MedRxiv
Summary by Cheleka AM Mpande










